Abstract
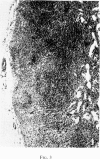
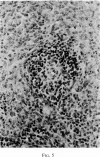
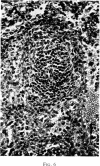
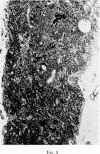
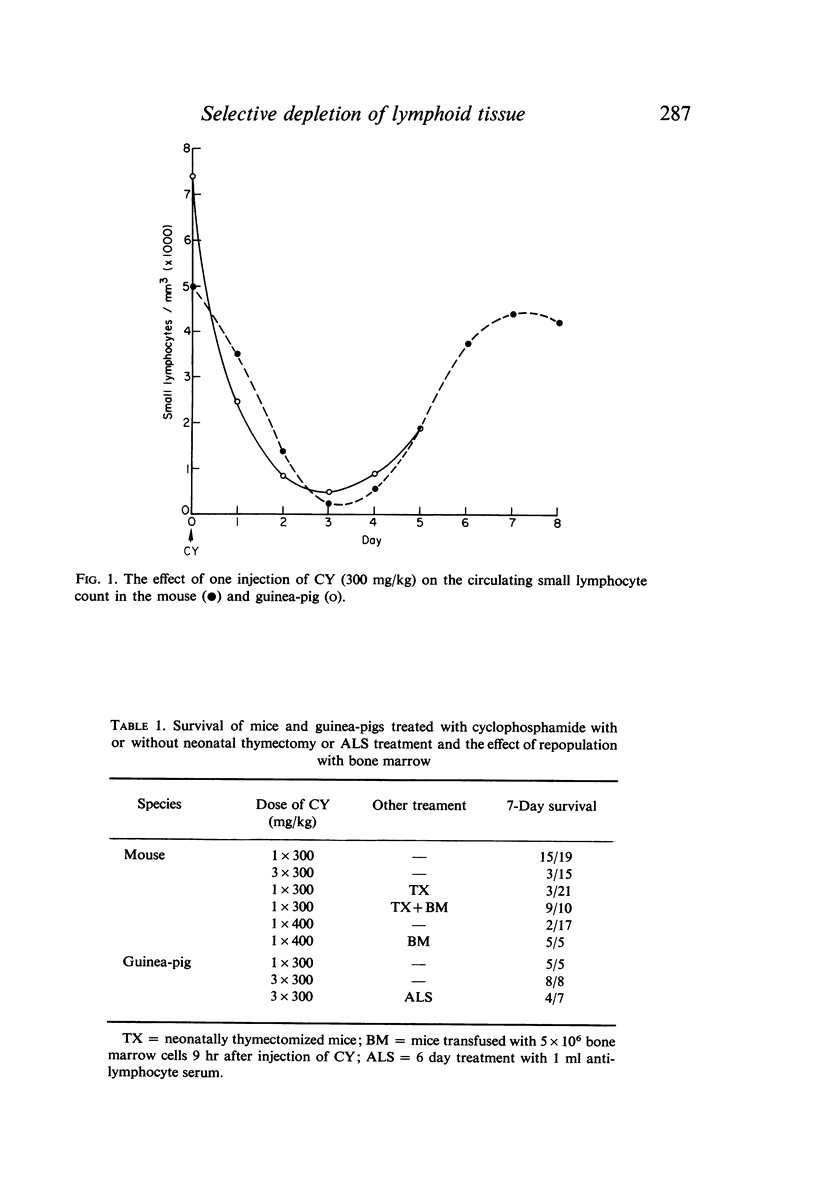
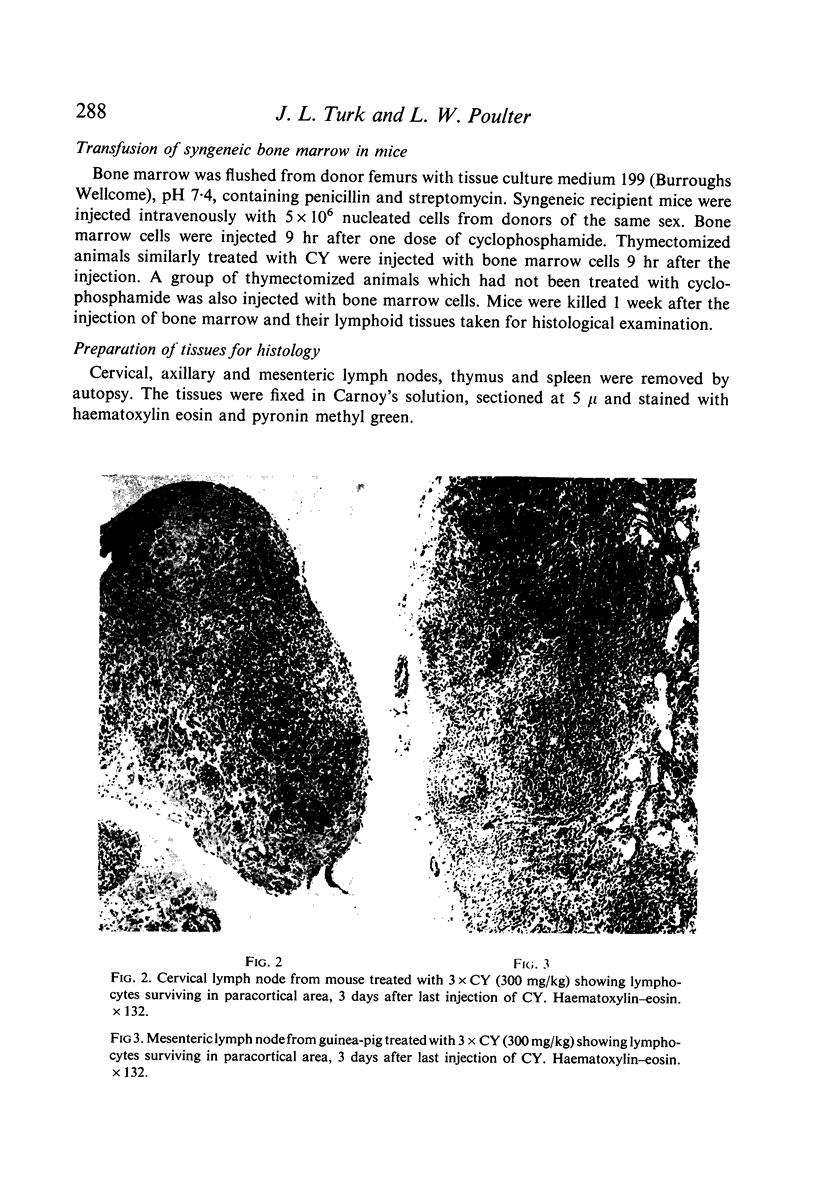
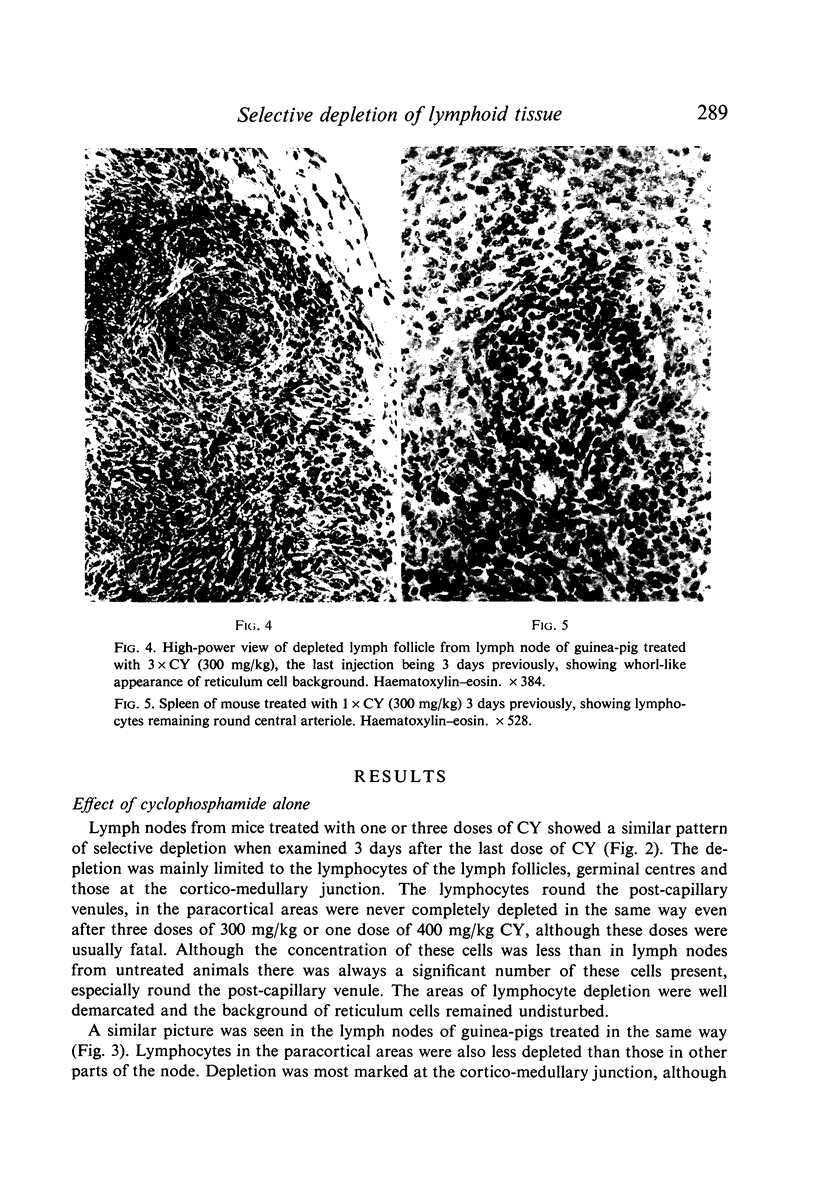
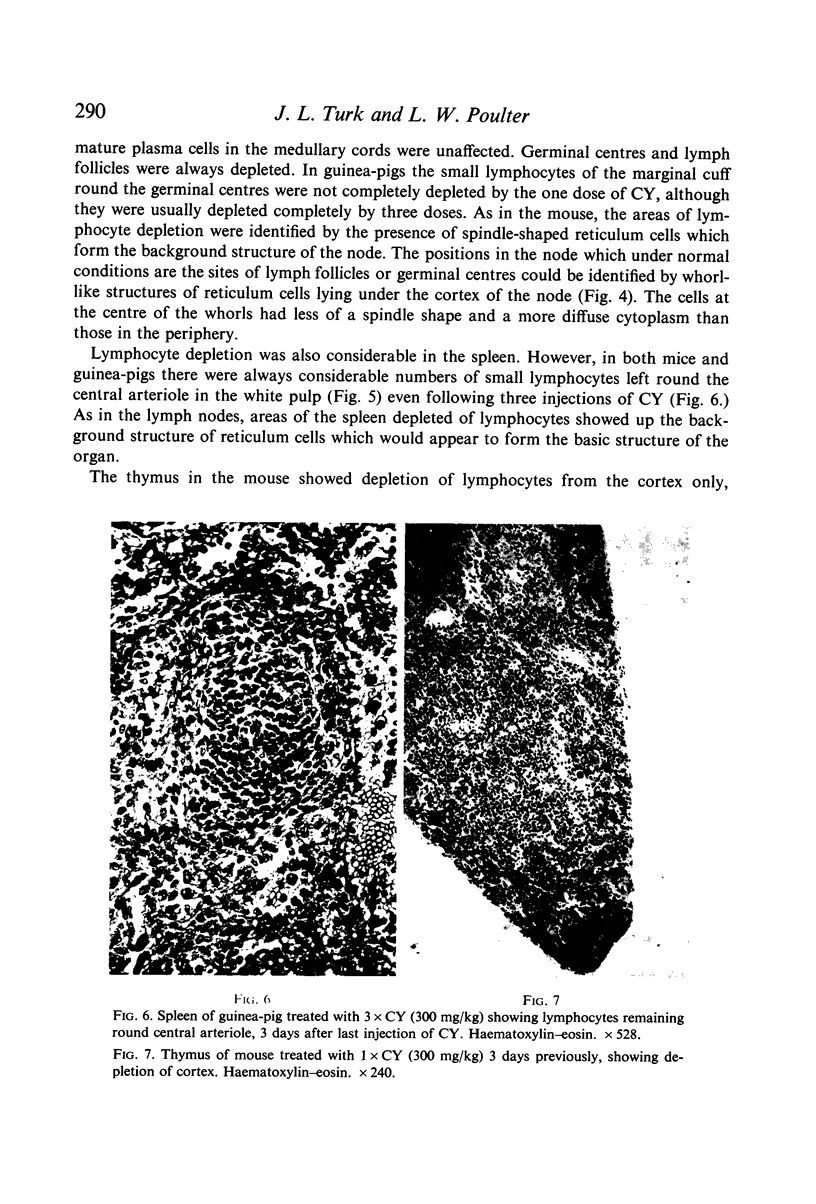
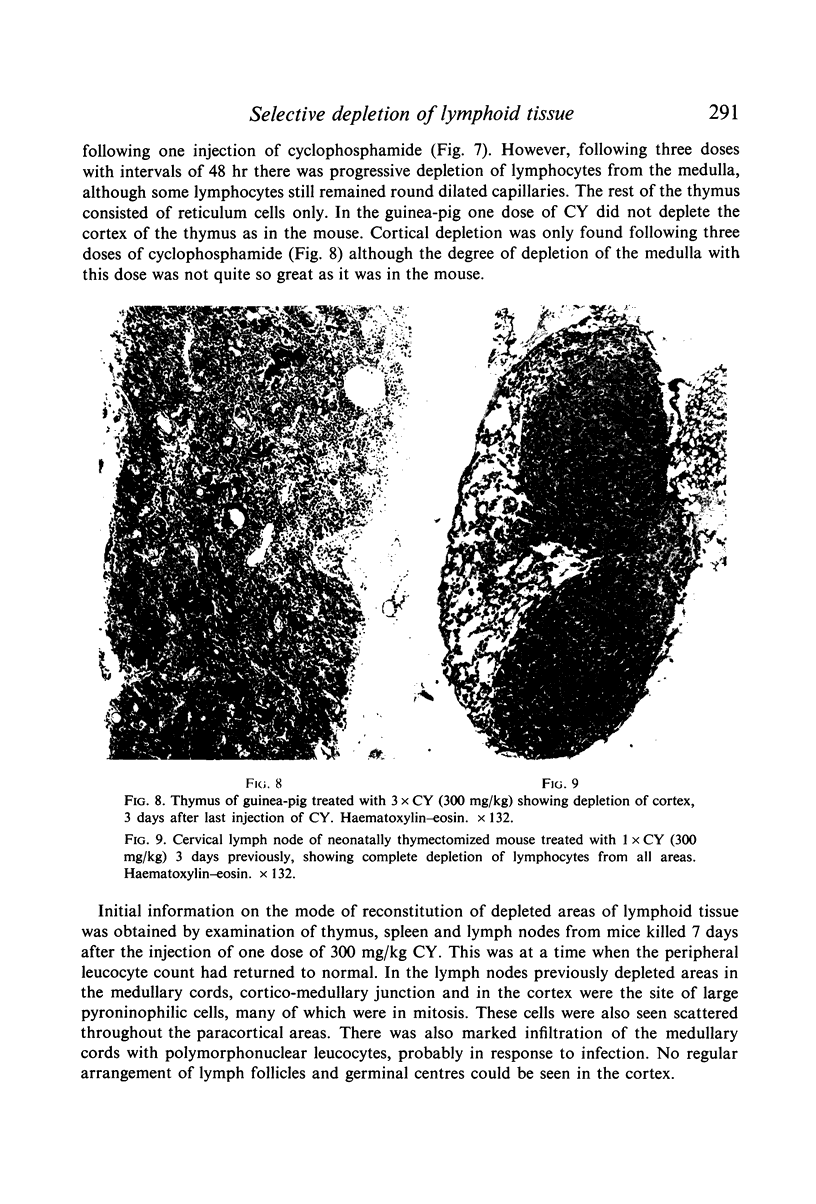
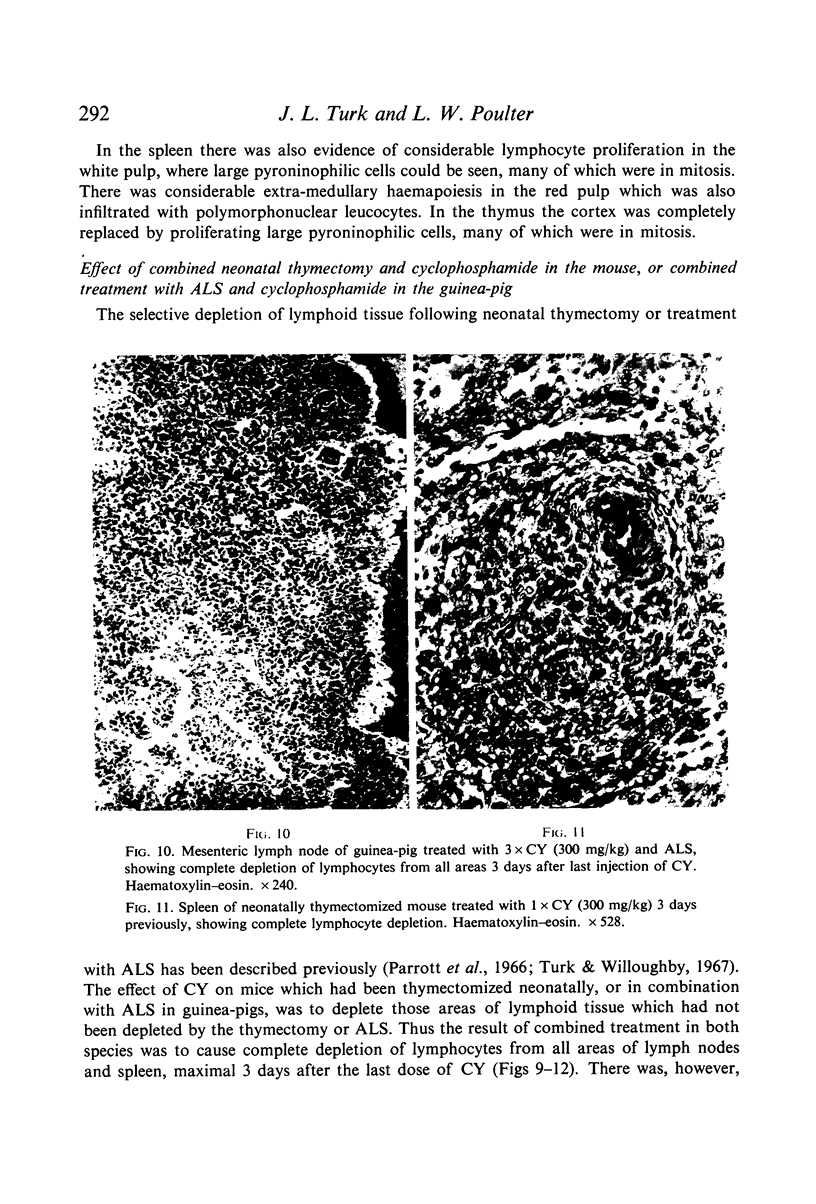
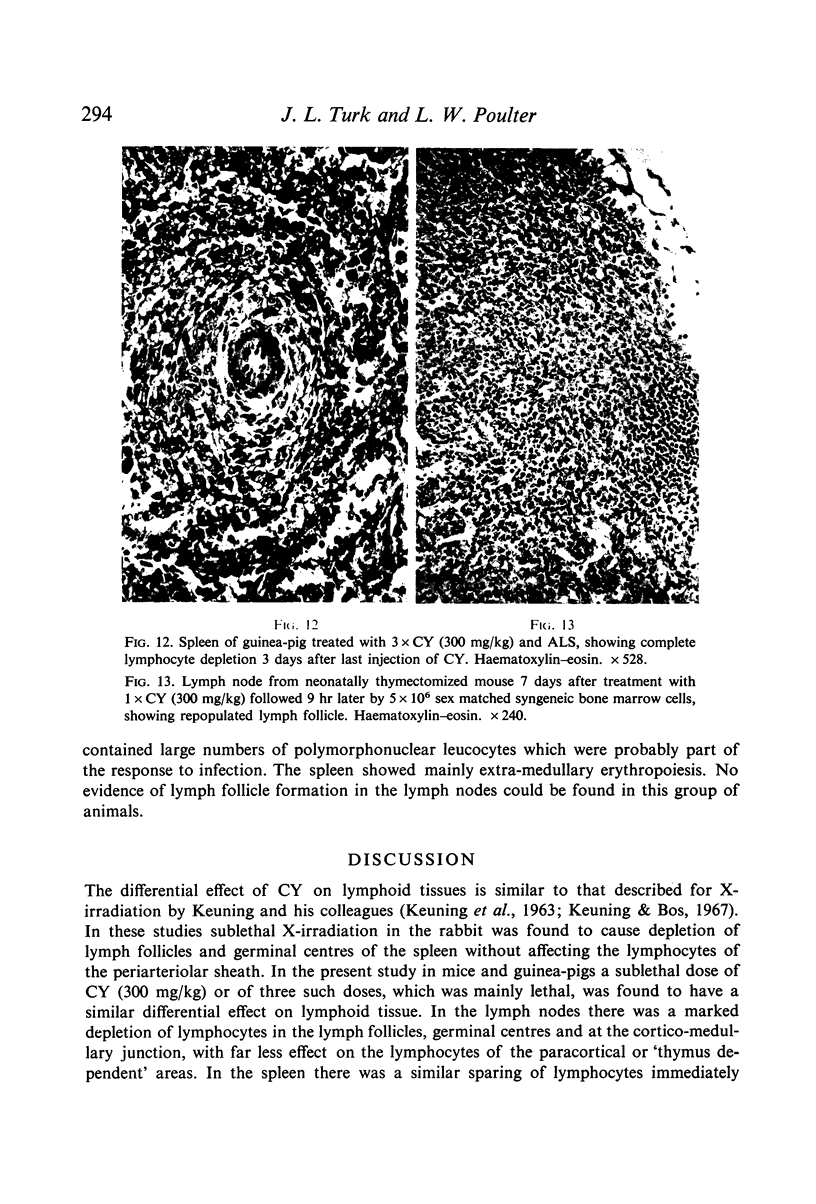
Selective depletion of lymphocytes from the lymph follicles and cortico-medullary junction in lymph nodes and equivalent non thymus dependent areas of the spleen can be produced by cyclophosphamide (CY) (300 mg/kg) in the mouse and guinea-pig. Despite three such injections on alternate days, thymus dependent areas still contained lymphocytes. Total depletion of lymphocytes from lymph nodes and spleen was produced by combining neonatal thymectomy in the mouse or ALS treatment in the guinea-pig with CY. CY produced depletion of lymphocytes in the cortex of the thymus before the medulla. Maximal depletion occurred at 3 days and in surviving animals repopulation was evident by 7 days at the cortico-medullary junction only. Lymph follicles were found in lymph nodes of neonatally thymectomized CY treated mice following repopulation with bone marrow. These findings suggest that the lymphocytes of the lymph follicles are derived from a population of rapidly dividing cells, part of which at least can be found in the bone marrow.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Denman E. J., Embling P. H. Changes in the life-span of circulating small lymphocytes in mice after treatment with anti-lymphocyte globulin. Lancet. 1968 Feb 17;1(7538):321–325. doi: 10.1016/s0140-6736(68)90791-5. [DOI] [PubMed] [Google Scholar]
- KEUNING F. J., van der MEER, NIEUWENHUIS P., OUDENDIJK P. The histophysiology of the antibody response. II. Antibody responses and splenic plasma cell reaction in sublethally x-irradiated rabbits. Lab Invest. 1963 Feb;12:156–170. [PubMed] [Google Scholar]
- Martin W. J., Miller J. F. Cell to cell interaction in the immune response. IV. Site of action of antilymphocyte globulin. J Exp Med. 1968 Oct 1;128(4):855–874. doi: 10.1084/jem.128.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OORT J., TURK J. L. A HISTOLOGICAL AND AUTORADIOGRAPHIC STUDY OF LYMPH NODES DURING THE DEVELOPMENT OF CONTACT SENSITIVITY IN THE GUINEA-PIG. Br J Exp Pathol. 1965 Apr;46:147–154. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov R. V., Manyko V. M., Khaitov R. M., Seslavina L. S. An experimental system for the simultaneous estimation of mitostatic and lymphotoxic effects of immunosuppressants and cytostatics. J Exp Med. 1971 Mar 1;133(3):640–648. doi: 10.1084/jem.133.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWELL O. A. Radiosensitivity of the cortical and medullary lymphocytes in the thymus. Int J Radiat Biol Relat Stud Phys Chem Med. 1961 Nov;4:163–173. doi: 10.1080/09553006114551091. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Willoughby D. A. Central and peripheral effects of anti-lymphocyte sera. Lancet. 1967 Feb 4;1(7484):249–251. doi: 10.1016/s0140-6736(67)91307-4. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Willoughby D. A., Stevens J. E. An analysis of the effects of some types of anti-lymphocyte sera on contact hypersensitivity and certain models of inflammation. Immunology. 1968 May;14(5):683–695. [PMC free article] [PubMed] [Google Scholar]