Abstract
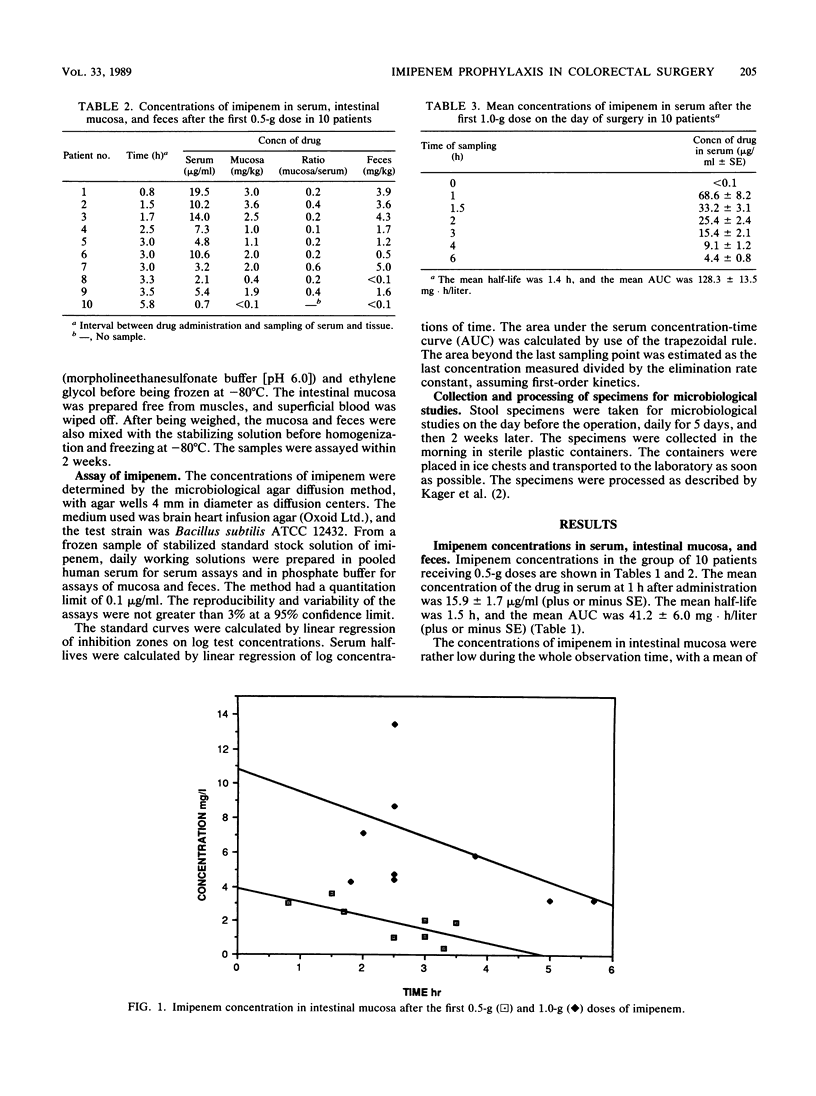
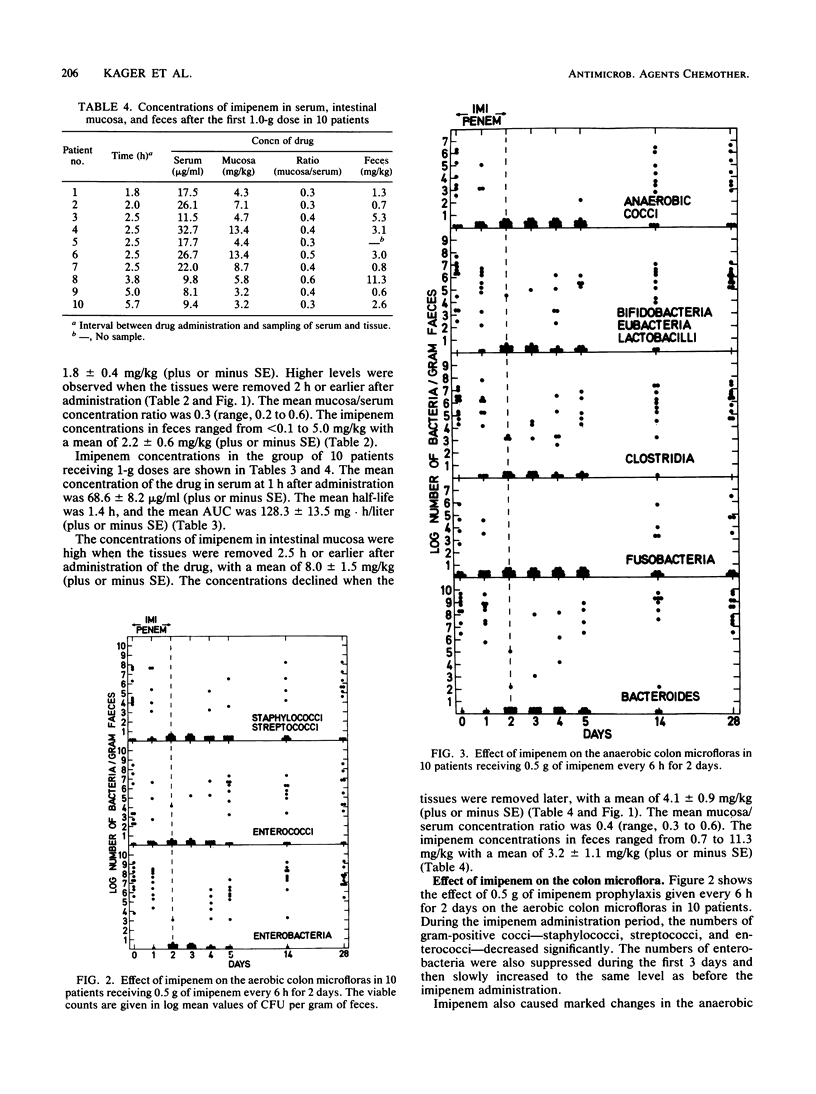
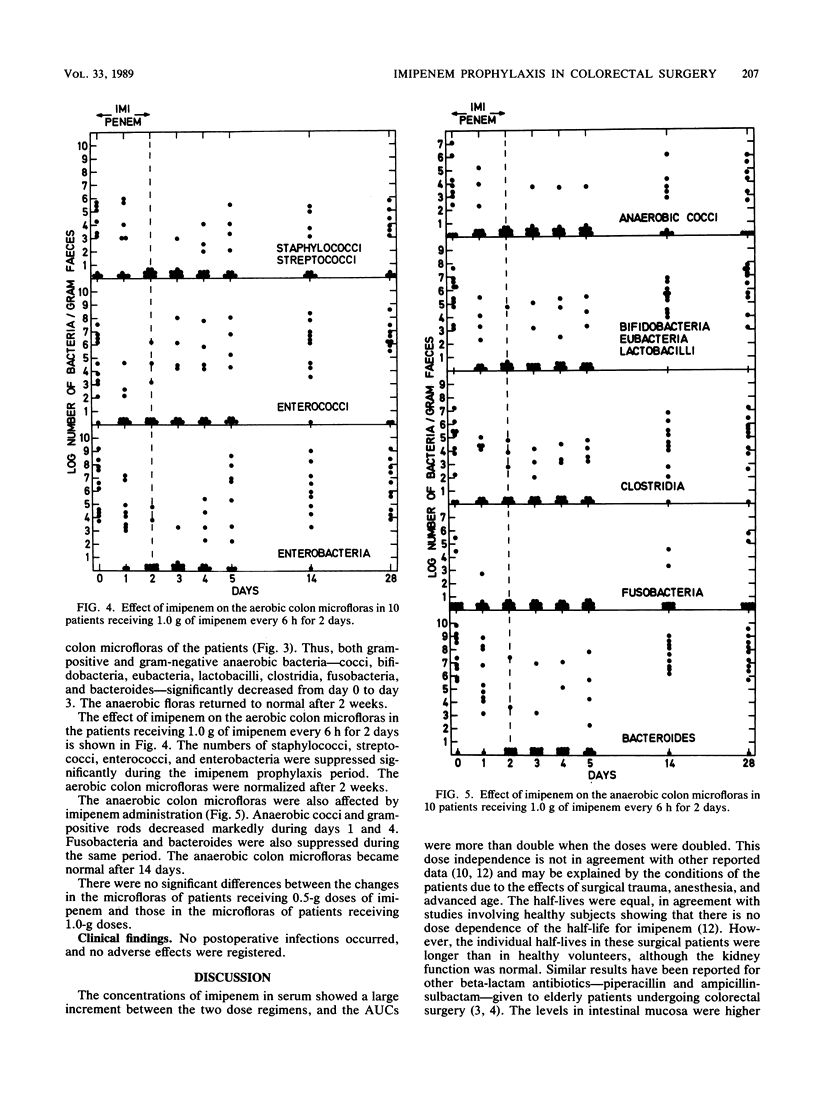
Twenty patients undergoing colorectal surgery were given, as prophylaxis, imipenem-cilastatin intravenously. Ten of them received a dose of 0.5/0.5 g of imipenem-cilastatin at induction of anesthesia, followed by subsequent doses of 0.5/0.5 g every 6 h for 48 h. The other 10 patients were given 1.0/1.0 g imipenem-cilastatin in the same way for 48 h. Samples from serum, intestinal mucosa, and feces were taken for analysis of imipenem concentrations during the day of surgery. The mean concentrations in serum at 1 h after the first imipenem dose were 15.9 +/- 1.7 micrograms/ml for the 0.5-g dose and 68.2 +/- 8.2 micrograms/ml for the 1.0-g dose. The mean half-lives were 1.5 and 1.4 h, respectively, and the mean areas under the serum concentration-time curve were 41.2 +/- 6.0 and 128.3 +/- 13.5 mg.h/liter, respectively. The imipenem concentrations in the intestinal mucosa varied between less than 0.1 and 3.6 mg/kg for the 0.5-g dose and 3.2 and 13.4 mg/kg for the 1.0-g dose. The concentrations in the fecal samples varied between less than 0.1 and 5.0 mg/kg for the 0.5-g dose and 0.7 and 11.3 mg/kg for the 1.0-g dose. Fecal samples were also collected during the investigation period for cultivation of aerobic and anaerobic bacteria. The aerobic bacteria--staphylococci, streptococci, enterococci, and enteroaerobic enterococci, and enterobacteria--were and anaerobic bacteria. The aerobic bacteria--staphylococci, streptococci, enterococci, and enterobacteria--were suppressed significantly during the imipenem prophylaxis period. Among the anaerobic bacteria, cocci, bifidobacteria, eubacteria, lactobacilli, clostridia, fusobacteria, and bacteroides decreased markedly during the same period. The microfloras were normalized after 2 weeks. There were no differences between the patients receiving 0.5-g doses of imipenem and those receiving 1.0-g of imipenem. No postoperative infections occurred.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gartell P. C., Sutton G. L., Eardley I., Porte M., Taylor E. A. Imipenem pharmacokinetics in elective colorectal surgery. J Antimicrob Chemother. 1986 Dec;18 (Suppl E):109–113. doi: 10.1093/jac/18.supplement_e.109. [DOI] [PubMed] [Google Scholar]
- Kager L., Ljungdahl I., Malmborg A. S., Nord C. E., Pieper R., Dahlgren P. Antibiotic prophylaxis with cefoxitin in colorectal surgery: effect on the colon microflora and septic complications--a clinical model for prediction of the benefit and risks in using a new antibiotic in prophylaxis. Ann Surg. 1981 Mar;193(3):277–282. doi: 10.1097/00000658-198103000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kager L., Malmborg A. S., Nord C. E., Sjöstedt S. The effect of piperacillin prophylaxis on the colonic microflora In patients undergoing colorectal surgery. Infection. 1983 Sep-Oct;11(5):251–254. doi: 10.1007/BF01641255. [DOI] [PubMed] [Google Scholar]
- Kager L., Malmborg A. S., Sjöstedt S., Nord C. E. Concentrations of ampicillin plus sulbactam in serum and intestinal mucosa and effects on the colonic microflora in patients undergoing colorectal surgery. Eur J Clin Microbiol. 1983 Dec;2(6):559–563. doi: 10.1007/BF02016565. [DOI] [PubMed] [Google Scholar]
- Kager L., Nord C. E. Imipenem/cilastatin in the treatment of intraabdominal infections: a review of worldwide experience. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S518–S521. doi: 10.1093/clinids/7.supplement_3.s518. [DOI] [PubMed] [Google Scholar]
- LJUNGQVIST U. WOUND SEPSIS AFTER CLEAN OPERATIONS. Lancet. 1964 May 16;1(7342):1095–1097. doi: 10.1016/s0140-6736(64)91291-7. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Gibson G. A., Bland J. A. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high-dose treatment for serious infections. Antimicrob Agents Chemother. 1986 Feb;29(2):188–192. doi: 10.1128/aac.29.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Heimdahl A., Kager L. Antimicrobial induced alterations of the human oropharyngeal and intestinal microflora. Scand J Infect Dis Suppl. 1986;49:64–72. [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Meisinger M. A., Rogers J. D. Pharmacokinetics and tolerance of N-formimidoyl thienamycin (MK0787) in humans. Antimicrob Agents Chemother. 1983 Feb;23(2):293–299. doi: 10.1128/aac.23.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Rogers J. D., Ferber F., Jones K. H., Zacchei A. G., Weidner L. L., Demetriades J. L., Gravallese D. A., Hsieh J. Y. Disposition of radiolabeled imipenem and cilastatin in normal human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):707–714. doi: 10.1128/aac.26.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. D., Meisinger M. A., Ferber F., Calandra G. B., Demetriades J. L., Bland J. A. Pharmacokinetics of imipenem and cilastatin in volunteers. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S435–S446. doi: 10.1093/clinids/7.supplement_3.s435. [DOI] [PubMed] [Google Scholar]


