Abstract
The fragile X syndrome is due to the new class of dynamic mutations. It is associated with an expansion of a trinucleotide repeat (CGG) in exon 1 of the fragile X mental retardation gene 1 gene (FMR1). Here we present a fragile X family with an unique female patient who was rendered hemizygous for the FRAXA locus due to a large deletion of one X chromosome. In addition, the other X had a microdeletion in FMR1. PCR and sequence analysis revealed that the microdeletion included all CGG repeats plus 97 bp of flanking sequences, leaving transcription start site and translation start site intact. Despite this total lack of CGG repeats in the FMR1 gene, Western blot analysis showed expression of FMRP, and the patient's phenotype was essentially normal. X-inactivation studies of the androgen-receptor (AR) locus and haplotype determination of microsatellite markers gave evidence that the deletion probably originated from regression of a fully mutated FMR1 gene. Although the minimal number of CGG repeats hitherto reported in FRAXA is six, and at least four other genes associated with CGG repeats are known, suggesting an as yet unknown function of these repeats, our study clearly demonstrates that the absence of CGG repeats does not abolish expression of the FMR1 gene in lymphoblastoid cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birot A. M., Delobel B., Gronnier P., Bonnet V., Maire I., Bozon D. A 5-megabase familial deletion removes the IDS and FMR-1 genes in a male Hunter patient. Hum Mutat. 1996;7(3):266–268. doi: 10.1002/(SICI)1098-1004(1996)7:3<266::AID-HUMU12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Houck G. E., Jr, Ding X., Zhong N., Nolin S., Glicksman A., Dobkin C., Jenkins E. C. Reverse mutations in the fragile X syndrome. Am J Med Genet. 1996 Aug 9;64(2):287–292. doi: 10.1002/(SICI)1096-8628(19960809)64:2<287::AID-AJMG11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Wilson P. J., Morris C. P., Hopwood J. J., Richards R. I., Sutherland G. R., Ray P. N. Characterization of a deletion at Xq27-q28 associated with unbalanced inactivation of the nonmutant X chromosome. Am J Hum Genet. 1992 Aug;51(2):316–322. [PMC free article] [PubMed] [Google Scholar]
- Deissler H., Behn-Krappa A., Doerfler W. Purification of nuclear proteins from human HeLa cells that bind specifically to the unstable tandem repeat (CGG)n in the human FMR1 gene. J Biol Chem. 1996 Feb 23;271(8):4327–4334. doi: 10.1074/jbc.271.8.4327. [DOI] [PubMed] [Google Scholar]
- Fisch G. S., Snow K., Thibodeau S. N., Chalifaux M., Holden J. J., Nelson D. L., Howard-Peebles P. N., Maddalena A. The fragile X premutation in carriers and its effect on mutation size in offspring. Am J Hum Genet. 1995 May;56(5):1147–1155. [PMC free article] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gecz J., Gedeon A. K., Sutherland G. R., Mulley J. C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat Genet. 1996 May;13(1):105–108. doi: 10.1038/ng0596-105. [DOI] [PubMed] [Google Scholar]
- Gedeon A. K., Baker E., Robinson H., Partington M. W., Gross B., Manca A., Korn B., Poustka A., Yu S., Sutherland G. R. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. doi: 10.1038/ng0892-341. [DOI] [PubMed] [Google Scholar]
- Geerkens C., Just W., Vogel W. Deletions of Xq and growth deficit: a review. Am J Med Genet. 1994 Apr 1;50(2):105–113. doi: 10.1002/ajmg.1320500202. [DOI] [PubMed] [Google Scholar]
- Gu Y., Lugenbeel K. A., Vockley J. G., Grody W. W., Nelson D. L. A de novo deletion in FMR1 in a patient with developmental delay. Hum Mol Genet. 1994 Sep;3(9):1705–1706. doi: 10.1093/hmg/3.9.1705. [DOI] [PubMed] [Google Scholar]
- Gu Y., Shen Y., Gibbs R. A., Nelson D. L. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat Genet. 1996 May;13(1):109–113. doi: 10.1038/ng0596-109. [DOI] [PubMed] [Google Scholar]
- Hajra A., Collins F. S. Structure of the leukemia-associated human CBFB gene. Genomics. 1995 Apr 10;26(3):571–579. doi: 10.1016/0888-7543(95)80177-n. [DOI] [PubMed] [Google Scholar]
- Hirst M., Grewal P., Flannery A., Slatter R., Maher E., Barton D., Fryns J. P., Davies K. Two new cases of FMR1 deletion associated with mental impairment. Am J Hum Genet. 1995 Jan;56(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Jones C., Penny L., Mattina T., Yu S., Baker E., Voullaire L., Langdon W. Y., Sutherland G. R., Richards R. I., Tunnacliffe A. Association of a chromosome deletion syndrome with a fragile site within the proto-oncogene CBL2. Nature. 1995 Jul 13;376(6536):145–149. doi: 10.1038/376145a0. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Malter H. E., Iber J. C., Willemsen R., de Graaff E., Tarleton J. C., Leisti J., Warren S. T., Oostra B. A. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997 Feb;15(2):165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- Malzac P., Biancalana V., Voelckel M. A., Moncla A., Pellissier M. C., Boccaccio I., Mattei J. F. Unexpected inheritance of the (CGG)n trinucleotide expansion in a fragile X syndrome family. Eur J Hum Genet. 1996;4(1):8–12. doi: 10.1159/000472163. [DOI] [PubMed] [Google Scholar]
- Mannermaa A., Pulkkinen L., Kajanoja E., Ryynänen M., Saarikoski S. Deletion in the FMR1 gene in a fragile-X male. Am J Med Genet. 1996 Aug 9;64(2):293–295. doi: 10.1002/(SICI)1096-8628(19960809)64:2<293::AID-AJMG12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Meijer H., de Graaff E., Merckx D. M., Jongbloed R. J., de Die-Smulders C. E., Engelen J. J., Fryns J. P., Curfs P. M., Oostra B. A. A deletion of 1.6 kb proximal to the CGG repeat of the FMR1 gene causes the clinical phenotype of the fragile X syndrome. Hum Mol Genet. 1994 Apr;3(4):615–620. doi: 10.1093/hmg/3.4.615. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milà M., Castellví-Bel S., Sánchez A., Lázaro C., Villa M., Estivill X. Mosaicism for the fragile X syndrome full mutation and deletions within the CGG repeat of the FMR1 gene. J Med Genet. 1996 Apr;33(4):338–340. doi: 10.1136/jmg.33.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet E., Chateau C., Taillandier A., Simon-Bouy B., Serre J. L. Recurrent and unexpected segregation of the FMR1 CGG repeat in a family with fragile X syndrome. Hum Genet. 1996 Apr;97(4):512–515. doi: 10.1007/BF02267077. [DOI] [PubMed] [Google Scholar]
- Nancarrow J. K., Kremer E., Holman K., Eyre H., Doggett N. A., Le Paslier D., Callen D. F., Sutherland G. R., Richards R. I. Implications of FRA16A structure for the mechanism of chromosomal fragile site genesis. Science. 1994 Jun 24;264(5167):1938–1941. doi: 10.1126/science.8009225. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., Halley D. J. Complex behavior of simple repeats: the fragile X syndrome. Pediatr Res. 1995 Nov;38(5):629–637. doi: 10.1203/00006450-199511000-00001. [DOI] [PubMed] [Google Scholar]
- Parrish J. E., Oostra B. A., Verkerk A. J., Richards C. S., Reynolds J., Spikes A. S., Shaffer L. G., Nelson D. L. Isolation of a GCC repeat showing expansion in FRAXF, a fragile site distal to FRAXA and FRAXE. Nat Genet. 1994 Nov;8(3):229–235. doi: 10.1038/ng1194-229. [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Quan F., Zonana J., Gunter K., Peterson K. L., Magenis R. E., Popovich B. W. An atypical case of fragile X syndrome caused by a deletion that includes the FMR1 gene. Am J Hum Genet. 1995 May;56(5):1042–1051. [PMC free article] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Kozman H., Kremer E., Lynch M., Pritchard M., Yu S., Mulley J., Sutherland G. R. Fragile X syndrome: genetic localisation by linkage mapping of two microsatellite repeats FRAXAC1 and FRAXAC2 which immediately flank the fragile site. J Med Genet. 1991 Dec;28(12):818–823. doi: 10.1136/jmg.28.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Yu S., Sutherland G. R. Fragile X syndrome unstable element, p(CCG)n, and other simple tandem repeat sequences are binding sites for specific nuclear proteins. Hum Mol Genet. 1993 Sep;2(9):1429–1435. doi: 10.1093/hmg/2.9.1429. [DOI] [PubMed] [Google Scholar]
- Riggins G. J., Sherman S. L., Phillips C. N., Stock W., Westbrook C. A., Warren S. T. CGG-repeat polymorphism of the BCR gene rules out predisposing alleles leading to the Philadelphia chromosome. Genes Chromosomes Cancer. 1994 Feb;9(2):141–144. doi: 10.1002/gcc.2870090211. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Do sequences in Xq27.3 play a role in X inactivation? 1992 Apr 15-May 1Am J Med Genet. 43(1-2):279–281. doi: 10.1002/ajmg.1320430143. [DOI] [PubMed] [Google Scholar]
- Tarleton J., Richie R., Schwartz C., Rao K., Aylsworth A. S., Lachiewicz A. An extensive de novo deletion removing FMR1 in a patient with mental retardation and the fragile X syndrome phenotype. Hum Mol Genet. 1993 Nov;2(11):1973–1974. doi: 10.1093/hmg/2.11.1973. [DOI] [PubMed] [Google Scholar]
- Trottier Y., Imbert G., Poustka A., Fryns J. P., Mandel J. L. Male with typical fragile X phenotype is deleted for part of the FMR1 gene and for about 100 kb of upstream region. Am J Med Genet. 1994 Jul 15;51(4):454–457. doi: 10.1002/ajmg.1320510431. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vits L., De Boulle K., Reyniers E., Handig I., Darby J. K., Oostra B., Willems P. J. Apparent regression of the CGG repeat in FMR1 to an allele of normal size. Hum Genet. 1994 Nov;94(5):523–526. doi: 10.1007/BF00211019. [DOI] [PubMed] [Google Scholar]
- Väisänen M. L., Haataja R., Leisti J. Decrease in the CGGn trinucleotide repeat mutation of the fragile X syndrome to normal size range during paternal transmission. Am J Hum Genet. 1996 Sep;59(3):540–546. [PMC free article] [PubMed] [Google Scholar]
- Wöhrle D., Hennig I., Vogel W., Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993 Jun;4(2):140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kotzot D., Hirst M. C., Manca A., Korn B., Schmidt A., Barbi G., Rott H. D., Poustka A., Davies K. E. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Yano-Yanagisawa H., Li Y., Wang H., Kohwi Y. Single-stranded DNA binding proteins isolated from mouse brain recognize specific trinucleotide repeat sequences in vitro. Nucleic Acids Res. 1995 Jul 25;23(14):2654–2660. doi: 10.1093/nar/23.14.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaff E., Rouillard P., Willems P. J., Smits A. P., Rousseau F., Oostra B. A. Hotspot for deletions in the CGG repeat region of FMR1 in fragile X patients. Hum Mol Genet. 1995 Jan;4(1):45–49. doi: 10.1093/hmg/4.1.45. [DOI] [PubMed] [Google Scholar]
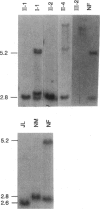
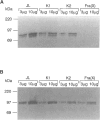
- de Graaff E., de Vries B. B., Willemsen R., van Hemel J. O., Mohkamsing S., Oostra B. A., van den Ouweland A. M. The fragile X phenotype in a mosaic male with a deletion showing expression of the FMR1 protein in 28% of the cells. Am J Med Genet. 1996 Aug 9;64(2):302–308. doi: 10.1002/(SICI)1096-8628(19960809)64:2<302::AID-AJMG14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]