Abstract
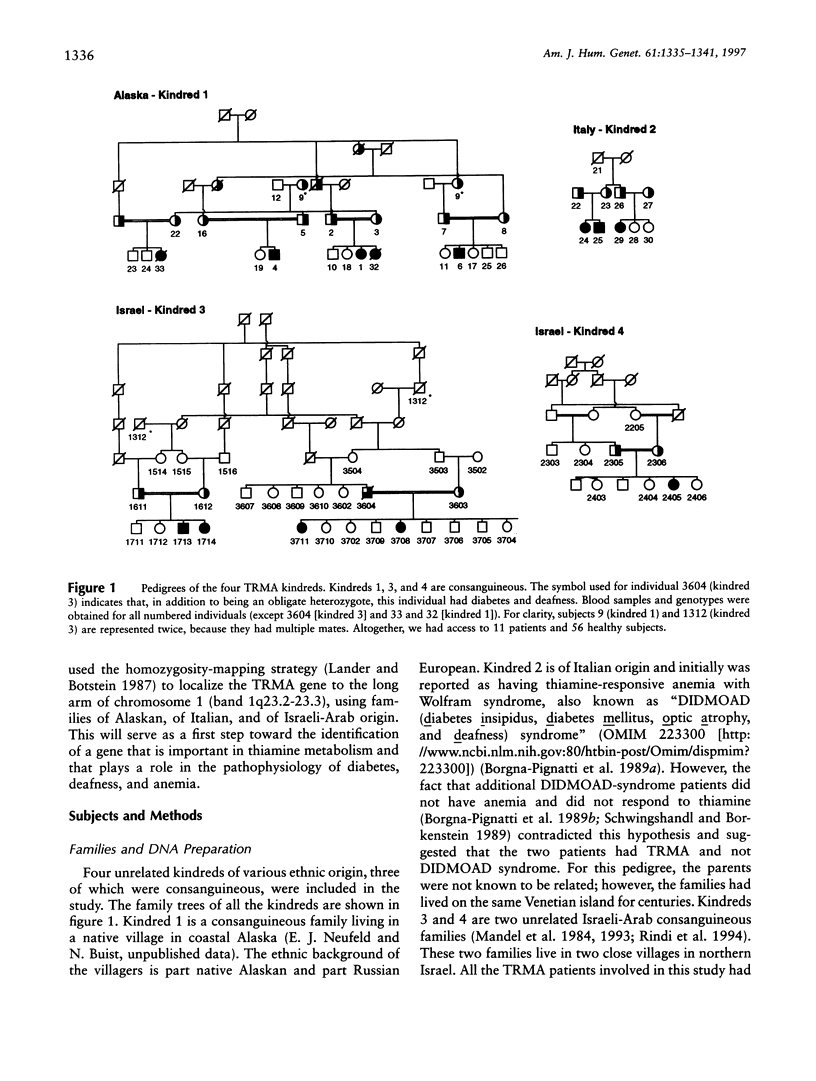
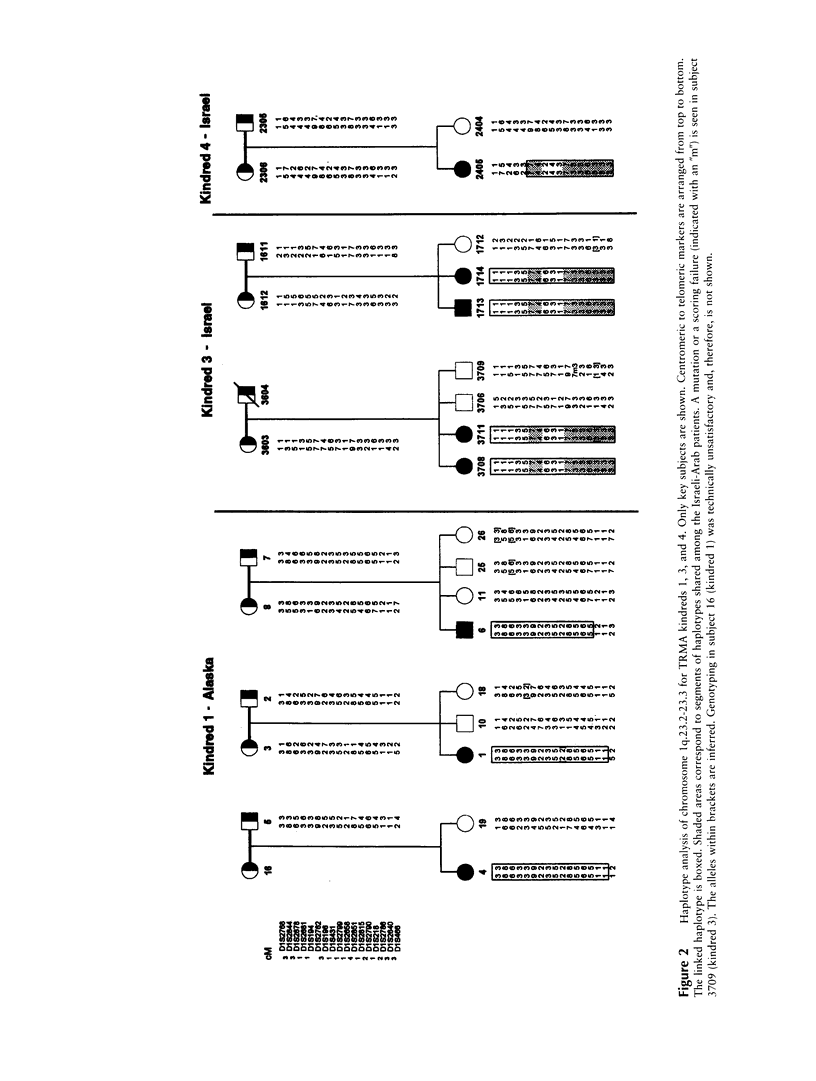
Thiamine-responsive megaloblastic anemia, also known as "TRMA" or "Rogers syndrome," is an early-onset autosomal recessive disorder defined by the occurrence of megaloblastic anemia, diabetes mellitus, and sensorineural deafness, responding in varying degrees to thiamine treatment. On the basis of a linkage analysis of affected families of Alaskan and of Italian origin, we found, using homozygosity mapping, that the TRMA-syndrome gene maps to a region on chromosome 1q23.2-23.3 (maximum LOD score of 3.7 for D1S1679). By use of additional consanguineous kindreds of Israeli-Arab origin, the putative disease-gene interval also has been confirmed and narrowed, suggesting genetic homogeneity. Linkage analysis generated the highest combined LOD-score value, 8.1 at a recombination fraction of 0, with marker D1S2799. Haplotype analysis and recombination events narrowed the TRMA locus to a 16-cM region between markers D1S194 and D1S2786. Several heterozygote parents had diabetes mellitus, deafness, or megaloblastic anemia, which raised the possibility that mutations at this locus predispose carriers in general to these manifestations. Characterization of the metabolic defect of TRMA may shed light on the role of thiamine deficiency in such common diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud M. R., Alexander D., Najjar S. S. Diabetes mellitus, thiamine-dependent megaloblastic anemia, and sensorineural deafness associated with deficient alpha-ketoglutarate dehydrogenase activity. J Pediatr. 1985 Oct;107(4):537–541. doi: 10.1016/s0022-3476(85)80011-1. [DOI] [PubMed] [Google Scholar]
- Akinci A., Teziç T., Ertürk G., Tarim O., Dalva K. Thiamine-responsive megaloblastic anemia with diabetes mellitus and sensorineural deafness. Acta Paediatr Jpn. 1993 Jun;35(3):262–266. doi: 10.1111/j.1442-200x.1993.tb03049.x. [DOI] [PubMed] [Google Scholar]
- Athma P., Rappaport R., Swift M. Molecular genotyping shows that ataxia-telangiectasia heterozygotes are predisposed to breast cancer. Cancer Genet Cytogenet. 1996 Dec;92(2):130–134. doi: 10.1016/s0165-4608(96)00328-7. [DOI] [PubMed] [Google Scholar]
- Barrientos A., Volpini V., Casademont J., Genís D., Manzanares J. M., Ferrer I., Corral J., Cardellach F., Urbano-Márquez A., Estivill X. A nuclear defect in the 4p16 region predisposes to multiple mitochondrial DNA deletions in families with Wolfram syndrome. J Clin Invest. 1996 Apr 1;97(7):1570–1576. doi: 10.1172/JCI118581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgna-Pignatti C., Marradi P., Pinelli L., Monetti N., Patrini C. Thiamine-responsive anemia in DIDMOAD syndrome. J Pediatr. 1989 Mar;114(3):405–410. doi: 10.1016/s0022-3476(89)80558-x. [DOI] [PubMed] [Google Scholar]
- Cagianut B., Hochstrasser P., Rhyner K. Diabetes juvenilis, tapeto-retinale Degeneration, neurogene Schwerhörigkeit (Alström-Syndrom), assoziiert mit einer kongenitalen dyserythropoietischen Anämie Typ III. Schweiz Med Wochenschr. 1977 Apr 2;107(13):446–450. [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Grill J., Leblanc T., Baruchel A., Daniel M. T., Dresch C., Schaison G. Thiamine responsive anemia: report of a new case associated with a thiamine pyrophosphokinase deficiency. Nouv Rev Fr Hematol. 1991;33(6):543–544. [PubMed] [Google Scholar]
- Haworth C., Evans D. I., Mitra J., Wickramasinghe S. N. Thiamine responsive anaemia: a study of two further cases. Br J Haematol. 1982 Apr;50(4):549–561. doi: 10.1111/j.1365-2141.1982.tb01955.x. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Kaitila I., Sistonen P., Weaver A., Lander E. Linkage disequilibrium mapping in isolated founder populations: diastrophic dysplasia in Finland. Nat Genet. 1992 Nov;2(3):204–211. doi: 10.1038/ng1192-204. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987 Jun 19;236(4808):1567–1570. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel H., Berant M., Hazani A., Naveh Y. Thiamine-dependent beriberi in the "thiamine-responsive anemia syndrome". N Engl J Med. 1984 Sep 27;311(13):836–838. doi: 10.1056/NEJM198409273111307. [DOI] [PubMed] [Google Scholar]
- Poggi V., Rindi G., Patrini C., De Vizia B., Longo G., Andria G. Studies on thiamine metabolism in thiamine-responsive megaloblastic anaemia. Eur J Pediatr. 1989 Jan;148(4):307–311. doi: 10.1007/BF00444120. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Swift R. G., Swift M. Linkage of the gene for Wolfram syndrome to markers on the short arm of chromosome 4. Nat Genet. 1994 Sep;8(1):95–97. doi: 10.1038/ng0994-95. [DOI] [PubMed] [Google Scholar]
- Porter F. S., Rogers L. E., Sidbury J. B., Jr Thiamine-responsive megaloblastic anemia. J Pediatr. 1969 Apr;74(4):494–504. doi: 10.1016/s0022-3476(69)80031-4. [DOI] [PubMed] [Google Scholar]
- Rindi G., Patrini C., Laforenza U., Mandel H., Berant M., Viana M. B., Poggi V., Zarra A. N. Further studies on erythrocyte thiamin transport and phosphorylation in seven patients with thiamin-responsive megaloblastic anaemia. J Inherit Metab Dis. 1994;17(6):667–677. doi: 10.1007/BF00712009. [DOI] [PubMed] [Google Scholar]
- Rosskamp R., Zigrahn W., Burmeister W. Thiaminabhängige Anämie und Thrombozytopenie, insulinpflichtiger Diabetes mellitus und sensorineurale Schwerhörigkeit--Fallbeschreibung und Ubersicht. Klin Padiatr. 1985 Jul-Aug;197(4):315–317. doi: 10.1055/s-2008-1033992. [DOI] [PubMed] [Google Scholar]
- Schwingshandl J., Borkenstein M. Treatment of DIDMOAD syndrome with thiamine. J Pediatr. 1989 Nov;115(5 Pt 1):834–834. doi: 10.1016/s0022-3476(89)80677-8. [DOI] [PubMed] [Google Scholar]
- Sirugo G., Keats B., Fujita R., Duclos F., Purohit K., Koenig M., Mandel J. L. Friedreich ataxia in Louisiana Acadians: demonstration of a founder effect by analysis of microsatellite-generated extended haplotypes. Am J Hum Genet. 1992 Mar;50(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Swift M., Morrell D., Massey R. B., Chase C. L. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991 Dec 26;325(26):1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- Viana M. B., Carvalho R. I. Thiamine-responsive megaloblastic anemia, sensorineural deafness, and diabetes mellitus: A new syndrome? J Pediatr. 1978 Aug;93(2):235–238. doi: 10.1016/s0022-3476(78)80503-4. [DOI] [PubMed] [Google Scholar]


