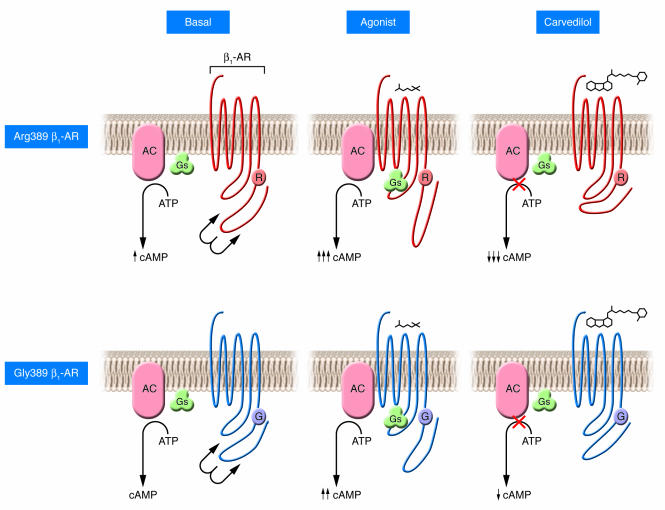
Figure 1. The effect of the β1-AR Gly389Arg polymorphism on signal transduction and beta blockade by carvedilol.
The Gly389Arg polymorphism of the β1-AR occurs in the region between the seventh transmembrane domain and the intracellular tail of the receptor (R and G correspond to arginine and glycine, respectively, at position 389 of the receptor). This highly conserved region is putatively associated with coupling to the Gs protein. The change of the amino acid residue at position 389 of the β1-AR from the polar, basic arginine (upper 3 panels) to the small, nonpolar glycine (lower 3 panels) may result in a modified structure that could alter receptor-Gs interaction. This region of the cytoplasmic tail of the receptor may participate in regulating the affinity of the receptor-Gs interaction. This could potentially explain the differences in receptor-Gs coupling and cAMP production associated with the Arg389 β1-AR variant as compared with the Gly389 β1-AR variant reported previously (7, 8). Under basal conditions, the Arg389 variant has been associated with increased cAMP production, which is markedly augmented as compared with the Gly389 variant following agonist stimulation (7). This effect could also be attributed to a more favorable structural environment for interaction with Gs. Carvedilol treatment induces a conformational change resulting in the intracellular tail of the receptor being positioned more closely to the third intracellular loop; this inverse agonism of the receptor in theory would lead to decreased Gs coupling and reduced cAMP accumulation. Both variants demonstrated inverse agonist properties in response to carvedilol; however, the effect was increased 2.5-fold in the Arg389 variant, apparently due to conformation of the Arg389 structure in the presence of the ligand as observed by Rochais et al. in this issue of the JCI (14).