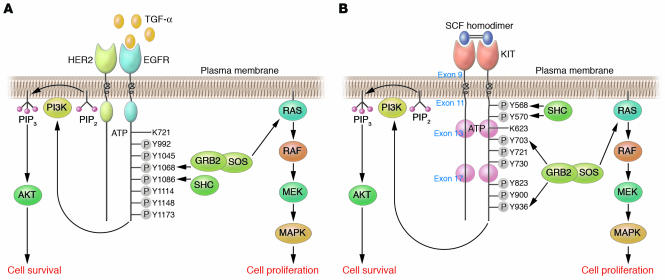
Figure 3. Schematic depiction of EGFR and KIT signaling.
(A) Expression of TGF-α is upregulated in the gastric mucosa of patients with Ménétrier disease. Ligand binding results in a conformational change in the ectodomain of the receptor from a tethered intramolecular state to a dimer-competent state (27). Homodimerization and/or heterodimerization with other EGFR family members results in activation of the intrinsic tyrosine kinase that phosphorylates, probably in trans, tyrosine residues (Y) in the cytoplasmic tail. Shown are the 7 autophosphorylation sites in the EGFR cytoplasmic tail. SH2-containing proteins bind these phosphorylated tyrosine residues to initiate a complex signaling cascade. GRB2 and SHC are reported to bind Y1068, Y1086, Y1148, and Y1173 (27). Activation or inhibition of EGFR usually results in coordinate regulation of MAPK and AKT, which are linked to proliferation and cell survival, respectively. (B) SCF is the only known KIT ligand. Binding of homodimeric SCF to KIT results in receptor homodimerization, activation of the intrinsic tyrosine kinase domain, and trans-phosphorylation of cytoplasmic KIT tyrosine residues. Somatic mutation of exon 9, 11, 13, or 17 in GISTs results in constitutive, ligand-independent KIT activation. Depicted are 8 tyrosine autophosphorylation sites in the KIT cytoplasmic domain. SH2-containing proteins bind these phosphorylated tyrosine residues and are then phosphorylated on tyrosine residues by KIT to initiate a complex signaling cascade. Y823 is located in the KIT activation loop; phosphorylation of this residue helps stabilize the activation loop in the “active” state and allows binding of protein substrates to the active site. Activation of KIT results in coordinate regulation of MAPK and AKT (108).