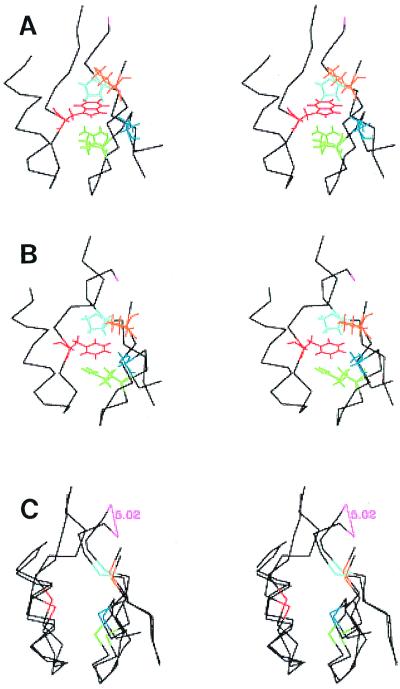
Figure 7.
Energy minimized neighborhood of the “responsive” tryptophan residue (Trp-512) and the “reactive” cysteine residue (Cys-717) of myosin in the presence of MgADP. This depiction is abstracted from Houdusse et al. (28), pdb1B7T, but known homology has been used to report sequence numeration as if it were that of the smooth muscle myosin used in this work. Trp-512 (red), Pro-722 (cyan), Asn-765 (blue), Arg-768 (orange), and Arg-777 (green) are identified by colors; only the α-carbon atoms of other residues are shown. (A) Wild type. (B) W512F mutant. (C) Regional structures of A and B arranged so that the foregoing five residues coincide. The rms separation distance for corresponding atoms is 1.42 Å. Note that the α-carbon of Arg-718 moves by 5 Å as a result of the mutation. The segment colored in pink shows this trajectory (beginning and end of the movement of the α-carbon).