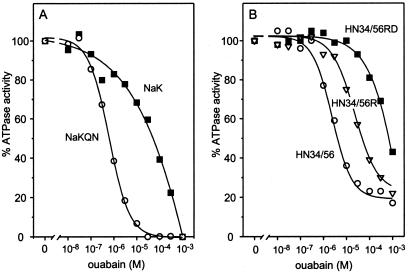
Figure 6.
Effect of ouabain on the ATPase activity of the rat Na+,K+-ATPase, mutant Na+,K+-ATPase R113Q/D124N, chimera HN34/56, mutant HN34/56 Q127R, and mutant HN34/56 Q127R/N138D. The assay shown in A was performed in the presence of 0.2 mM EDTA/0.1 mM EGTA/1.2 mM MgCl2/50 mM Tris-acetic acid, pH 7.0/10 mM KCl/100 mM NaCl/100 μM ATP. The assay conditions used for the assay shown in B were similar to those described in Fig. 2. The ATPase activity determined was corrected for that of the mock. The ATPase activity in the absence of ouabain was set at 100%: Na+,K+-ATPase, 0.37 μmol Pi mg−1⋅protein h−1 (■); NaKQN, 0.41 μmol Pi mg−1⋅protein h−1 (○); HN34/56, 0.10 μmol Pi mg−1⋅protein h−1 (○); HN34/56R, 0.08 μmol Pi mg−1⋅protein h−1 (▿); HN34/56RD, 0.04 μmol Pi mg−1⋅protein h−1 (■). Results are representative of two enzyme preparations.