Figure 2.
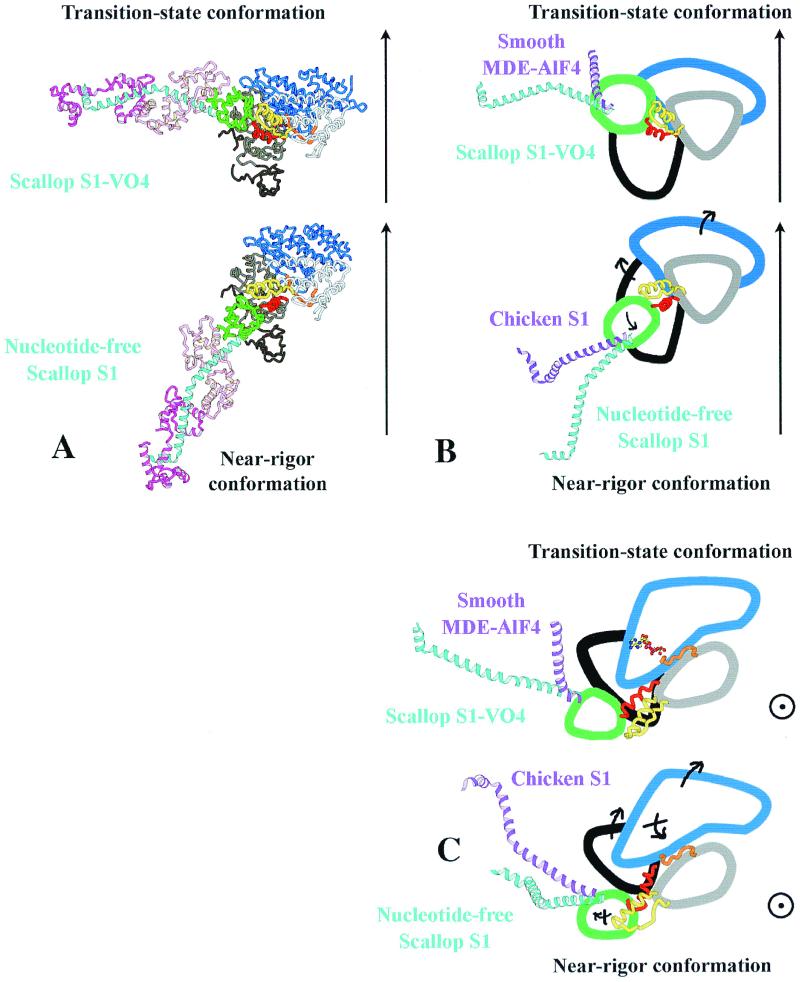
(A) Ribbon diagrams of the nucleotide-free scallop S1 structure (Lower) and of scallop S1-VO4 (Upper) oriented such that the lower 50-kDa subdomains of these two structures superimpose. An arrow indicates the approximate direction of the actin filament axis relative to this subdomain, deduced from an electron microscope study of S1-decorated actin (16). The position of the ELC in the scallop nucleotide-free structure is very close to that found in the electron-microscope maps of actin decorated with vertebrate smooth muscle myosin S1 under rigor conditions (16). No data are available to indicate how S1 binds to actin in the prepower stroke state; for illustrative purposes only, we have chosen to orient this structure by assuming that the interactions with the lower 50-kDa subdomain would be conserved. The lever arm is positioned at ≈90° and 25° to the actin filament axis in the transition-state and near-rigor structures, respectively. (Note that for measuring angles, the lever-arm position is taken as a straight line drawn from the N-terminal side of the lever-arm helix to the sharp bend near the C terminus.) (B) Schematic drawings of the transition-state and the near-rigor conformations of scallop myosin from an interpretation of the structures seen in A. The rotation of the converter (green)/relay (yellow) module during the power stroke is amplified by the lever arm (scallop blue helix, light chains omitted for clarity). The direction of the movement of the subdomains in the transition between the two states is indicated with black arrows. Although the subdomains of the MD are similar in different isoforms, differences are seen in the lever-arm position. To illustrate this point, the position of the lever arm found in smooth muscle MDE (purple helix, Upper) and that of chicken striated muscle myosin S1 (purple helix, Lower) is compared with the positions found for scallop myosin in the transition state and near-rigor state, respectively. Differences in the bending of the heavy-chain helix at the junction between the converter and the lever arm result in markedly different orientations for the lever arm of these structures representing the same state. (C) Schematic drawing of an orthogonal view of the structures seen in A. In this orientation, the actin filament axis is approximately perpendicular to the page, and one can thus estimate the azimuthal component of the movement of the lever arm. This component is very small in the case of scallop. In contrast, bending of the heavy-chain helix at the pliant region in smooth MDE in the transition-state conformation could lead to a large azimuthal component during the power-stroke in this myosin. Comparison of the transition-state and near-rigor conformations in this view reveals changes in the position of the upper and lower 50-kDa subdomains related to differences in both the conformation of switch II and the actin-binding site.