Abstract
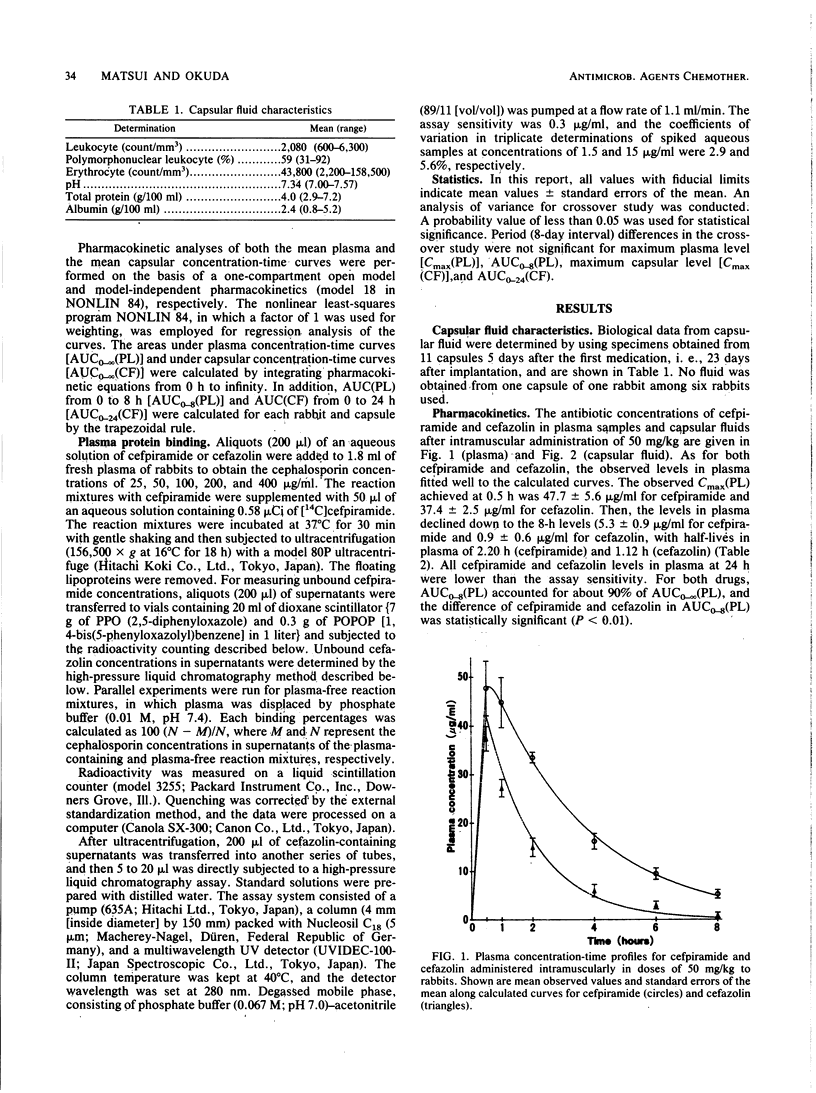
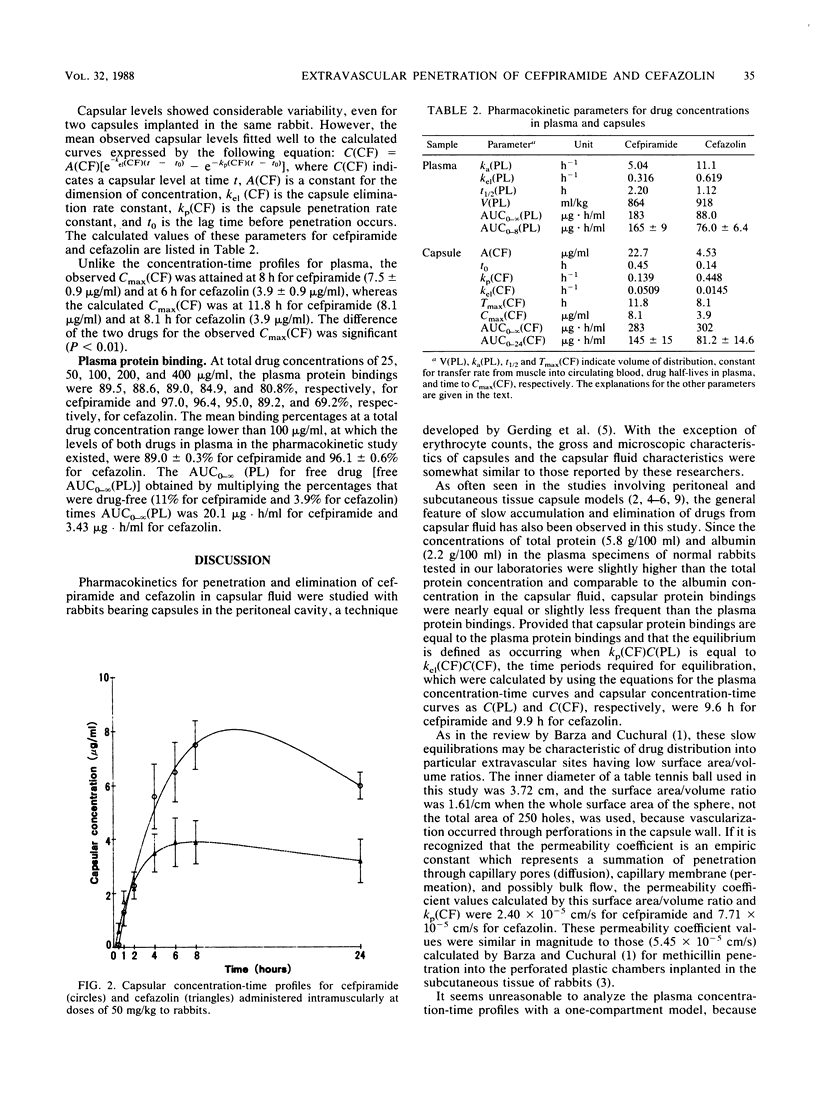
Penetration of cefpiramide and cefazolin into a specific extravascular fluid was measured with rabbits bearing capsules in the peritoneal cavity. A general feature of slow accumulation and elimination of drugs from extravascular sites having low surface area/volume ratios has also been observed in this study. The capsular concentration-time profiles were well expressed by the following equation: C(CF) = A(CF)[e-kel(CF)(t-to)-e-kp(CF)(t-to)], where C(CF), A(CF), kp(CF), kel(CF), and to indicate capsular concentration at time t, constant for the dimension of concentration, capsule penetration rate constant, capsule elimination rate constant, and lag time before penetration occurs, respectively. The kp(CF), kel(CF), and to were 0.139 h-1, 0.059 h-1, and 0.45 h, respectively, for cefpiramide, and 0.448 h-1, 0.0145 h-1, and 0.14 h, respectively, for cefazolin. A(CF) was 22.7 micrograms/ml for cefpiramide and 4.53 micrograms/ml for cefazolin, being parallel to the area under the plasma concentration-time curve for free drug from to to infinity (20.1 micrograms.h/ml for cefpiramide and 3.43 micrograms.h/ml for cefazolin). In conclusion, it is suggested that as well as kp(CF) and kel(CF), the area under the plasma concentration-time curve for free drug from to to infinity may play an important role regarding the circulating reservoir of drugs in determining capsular concentration-time profiles in experimental models for particular extravascular sites of infection, like abscesses into which drugs cannot easily penetrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M., Cuchural G. General principles of antibiotic tissue penetration. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):59–75. doi: 10.1093/jac/15.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Brion N., Lamotte-Barrillon S. Penetration of cefazolin, cephaloridine, and cefamandole into interstitial fluid in rabbits. Antimicrob Agents Chemother. 1977 Apr;11(4):594–598. doi: 10.1128/aac.11.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo F. M., Schentag J. J. Methicillin distribution in serum and extravascular fluid and its relevance to normal and damaged heart valves. Antimicrob Agents Chemother. 1981 May;19(5):836–841. doi: 10.1128/aac.19.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A., Schütze E. Concentrations of various antibiotics in serum and fluids accumulated in diffusion chambers implanted in various sites in rabbits. Antimicrob Agents Chemother. 1980 May;17(5):779–783. doi: 10.1128/aac.17.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Hall W. H., Schierl E. A., Manion R. E. Cephalosporin and aminoglycoside concentrations in peritoneal capsular fluid in rabbits. Antimicrob Agents Chemother. 1976 Dec;10(6):902–911. doi: 10.1128/aac.10.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Van Etta L. L., Peterson L. R. Role of serum protein binding and multiple antibiotic doses in the extravascular distribution of ceftizoxime and cefotaxime. Antimicrob Agents Chemother. 1982 Nov;22(5):844–847. doi: 10.1128/aac.22.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Yano K., Okuda T. Pharmacokinetics of the cephalosporin SM-1652 in mice, rats, rabbits, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1982 Aug;22(2):213–217. doi: 10.1128/aac.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Koyama M., Matsui H., Ikeda C., Yano K., Nakatsuru N., Yoshinaga K., Noguchi T. Pharmacokinetics of cefpiramide (SM-1652) in humans. Antimicrob Agents Chemother. 1984 Feb;25(2):221–225. doi: 10.1128/aac.25.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman N. G., Kastan L. B. Interstitial fluid and serum antibiotic concentrations. Arch Surg. 1972 Aug;105(2):192–196. doi: 10.1001/archsurg.1972.04180080046008. [DOI] [PubMed] [Google Scholar]


