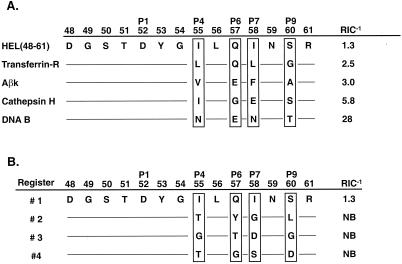
Figure 4.
Favorable and unfavorable auxiliary anchors affect peptide binding. (A) Favorable auxiliary anchors can be exchanged. The HEL(48-61) auxiliary anchors were replaced with those from four different natural I-Ak epitopes [transferrin receptor () KGTDFQLNQLEGKKGY (ref. 10), DNA B () DPFKGDDFNEENPTEPS (ref. 10), Aβk (37–53) YVRFDSDVGEERAVTEL (ref. 25), and cathepsin H (77–92) YILYNKGIMGEDSYPY (ref. 25)], all of which contained a similar strong P1 anchor, either Asp or Asn (23). The resulting hybrid peptides bound I-Ak with similar or improved RIC−1 values as the original natural peptides: transferrin-R RIC−1 = 14, Aβk = 2, cathepsin H = 23, and DNA B = 28. (B) HEL(48-61) auxiliary anchors were substituted with all four predicted anchors from each alternative register, 2, 3, or 4 (Fig. 1) and were tested for I-Ak binding. Replacement of HEL(48-61) auxiliary anchors (register 1) with those of registers 2, 3, or 4 completely blocked peptide binding, even though individual substitutions with each anchor had little effect on peptide binding.