Abstract
AIMS—To evaluate the incidence of loss of heterozygosity (LOH) and microsatellite instability (MI) in pterygia and their possible correlation with clinical variables. METHODS—50 pterygia, blood, and conjunctival specimens were obtained. A personal and family history was recorded for each patient. Amplification of 15 microsatellite markers at regions 17p, 17q, 13q, 9p, and 9q was performed using the polymerase chain reaction. The electrophoretic pattern of DNA from pterygia was compared with the respective pattern from blood and conjunctiva. RESULTS—LOH incidence was the highest at 9p (48%), followed by 17q (42%). Only three cases displayed MI. LOH incidence at individual markers was positively correlated with recurrence (D9S59, p=0.11 and D9S270, p=0.16), family history of neoplasia (D13S175, p=0.09), altitude of present residence ( D9S112, p=0.1), duration of the existence of pterygium (D9S144, p=0.06), and inversely correlated with age (D9S59, p=0.09). Concerning chromosome arms, LOH was positively correlated with the altitude of present residence (13q and 17p, p=0.03) and duration of the existence of pterygium (13q and 17p, p=0.09). CONCLUSIONS—LOH is a common event whereas MI is a very uncommon one at the examined markers in pterygium, indicating the presence of putative tumour suppressor genes implicated in the aetiopathogenesis of the disease. The fact that LOH at 9q31-33 was more frequent in recurrent pterygia and also correlated with known risk factors such as young age and high altitude of residence, implies a possible predictive value of this finding for postoperative recurrence. Keywords: heterozygosity; microsatellite instability; pterygium
Full Text
The Full Text of this article is available as a PDF (132.1 KB).
Figure 1 .
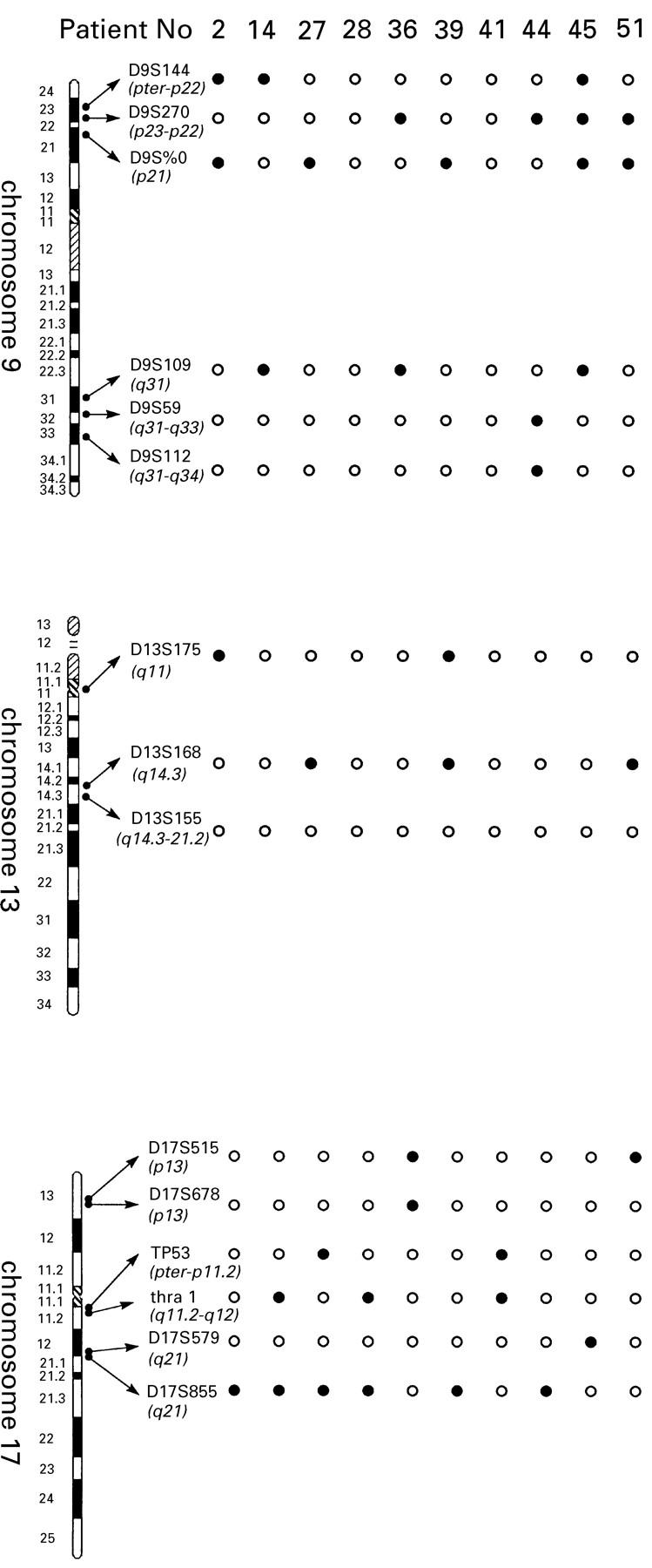
Location of selected markers at chromosome 9, 13, and 17 and loss of heterozygosity in 10 randomly selected cases (•, loss of heterozygosity; ∘, heterozygosity).
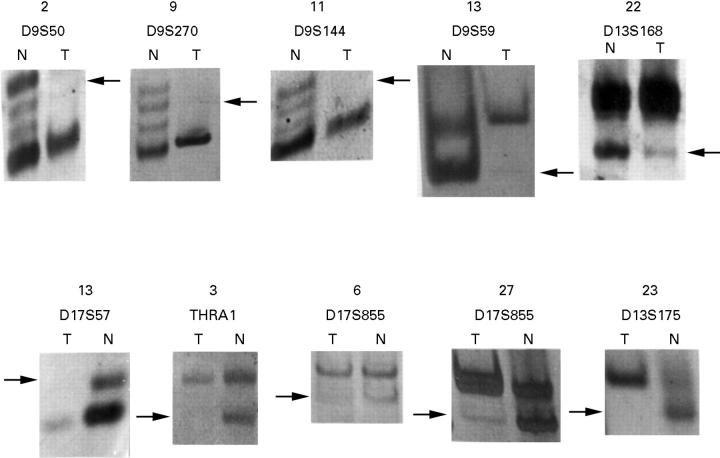
Figure 2 .
Representative samples of loss of heterozygosity detected in pterygium. N=normal DNA, T=pathological DNA. Arrows indicate the position of a deleted allele. Faint bands in the position of a deleted allele are interpreted as contamination by adjacent normal DNA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann M. W., Picard F., An H. X., van Roeyen C. R., Dominik S. I., Mosny D. S., Schnürch H. G., Bender H. G., Niederacher D. Clinical impact of detection of loss of heterozygosity of BRCA1 and BRCA2 markers in sporadic breast cancer. Br J Cancer. 1996 May;73(10):1220–1226. doi: 10.1038/bjc.1996.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquer-Maumont A., Crouau-Roy B. Polymorphism, monomorphism, and sequences in conserved microsatellites in primate species. J Mol Evol. 1995 Oct;41(4):492–497. doi: 10.1007/BF00160321. [DOI] [PubMed] [Google Scholar]
- Chaganti S. R., Gaidano G., Louie D. C., Dalla-Favera R., Chaganti R. S. Diffuse large cell lymphomas exhibit frequent deletions in 9p21-22 and 9q31-34 regions. Genes Chromosomes Cancer. 1995 Jan;12(1):32–36. doi: 10.1002/gcc.2870120106. [DOI] [PubMed] [Google Scholar]
- Chen J. K., Tsai R. J., Lin S. S. Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev Biol Anim. 1994 Apr;30A(4):243–248. doi: 10.1007/BF02632046. [DOI] [PubMed] [Google Scholar]
- Coroneo M. T. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993 Nov;77(11):734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Dunlop M. G., Wyllie A. H., Bird C. C. Deletion mapping in colorectal cancer of a putative tumour suppressor gene in 8p22-p21.3. Oncogene. 1993 May;8(5):1391–1396. [PubMed] [Google Scholar]
- Degrassi M., Piantanida A., Nucci P. Unexpected histological findings in pterygium. Optom Vis Sci. 1993 Dec;70(12):1058–1060. doi: 10.1097/00006324-199312000-00012. [DOI] [PubMed] [Google Scholar]
- Gailani M. R., Leffell D. J., Ziegler A., Gross E. G., Brash D. E., Bale A. E. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996 Mar 20;88(6):349–354. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- Gordon K. B., Thompson C. T., Char D. H., O'Brien J. M., Kroll S., Ghazvini S., Gray J. W. Comparative genomic hybridization in the detection of DNA copy number abnormalities in uveal melanoma. Cancer Res. 1994 Sep 1;54(17):4764–4768. [PubMed] [Google Scholar]
- HILGERS J. H. Pterygium: its incidence, heredity and etiology. Am J Ophthalmol. 1960 Oct;50:635–644. doi: 10.1016/0002-9394(60)90245-2. [DOI] [PubMed] [Google Scholar]
- Harding J. J. The untenability of the sunlight hypothesis of cataractogenesis. Doc Ophthalmol. 1994;88(3-4):345–349. doi: 10.1007/BF01203687. [DOI] [PubMed] [Google Scholar]
- Hecht F., Shoptaugh M. G. Winglets of the eye: dominant transmission of early adult pterygium of the conjunctiva. J Med Genet. 1990 Jun;27(6):392–394. doi: 10.1136/jmg.27.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimbo K. Traitement chirurgical du ptérygion: 42 cas d'excision. J Fr Ophtalmol. 1988;11(4):335–338. [PubMed] [Google Scholar]
- Kiaris H., Ergazaki M., Spandidos D. A. Instability at the H-ras minisatellite is associated with the spontaneous abortion of the embryo. Biochem Biophys Res Commun. 1995 Sep 25;214(3):788–792. doi: 10.1006/bbrc.1995.2355. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Ro J. Y., Kemp B. L., Lee J. S., Kwon T. J., Fong K. M., Sekido Y., Minna J. D., Hong W. K., Mao L. Identification of three distinct tumor suppressor loci on the short arm of chromosome 9 in small cell lung cancer. Cancer Res. 1997 Feb 1;57(3):400–403. [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Mackenzie F. D., Hirst L. W., Battistutta D., Green A. Risk analysis in the development of pterygia. Ophthalmology. 1992 Jul;99(7):1056–1061. doi: 10.1016/s0161-6420(92)31850-0. [DOI] [PubMed] [Google Scholar]
- Mao L., Lee D. J., Tockman M. S., Erozan Y. S., Askin F., Sidransky D. Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9871–9875. doi: 10.1073/pnas.91.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincione G. P., Taddei G. L., Wolovsky M., Calzolari A., Mincione F. Detection of human papillomavirus (HPV) DNA type 6/11 in a conjunctival papilloma by in situ hybridization with biotinylated probes. Pathologica. 1992 Jul-Aug;84(1092):483–488. [PubMed] [Google Scholar]
- Mitsudomi T., Oyama T., Nishida K., Ogami A., Osaki T., Sugio K., Yasumoto K., Sugimachi K., Gazdar A. F. Loss of heterozygosity at 3p in non-small cell lung cancer and its prognostic implication. Clin Cancer Res. 1996 Jul;2(7):1185–1189. [PubMed] [Google Scholar]
- Purdie C. A., Piris J., Bird C. C., Wyllie A. H. 17q allele loss is associated with lymph node metastasis in locally aggressive human colorectal cancer. J Pathol. 1995 Mar;175(3):297–302. doi: 10.1002/path.1711750307. [DOI] [PubMed] [Google Scholar]
- Quinn A. G., Sikkink S., Rees J. L. Delineation of two distinct deleted regions on chromosome 9 in human non-melanoma skin cancers. Genes Chromosomes Cancer. 1994 Dec;11(4):222–225. doi: 10.1002/gcc.2870110404. [DOI] [PubMed] [Google Scholar]
- Rehman I., Quinn A. G., Healy E., Rees J. L. High frequency of loss of heterozygosity in actinic keratoses, a usually benign disease. Lancet. 1994 Sep 17;344(8925):788–789. doi: 10.1016/s0140-6736(94)92343-4. [DOI] [PubMed] [Google Scholar]
- Rohrbach I. M., Starc S., Knorr M. Vorhersage von Pterygiumrezidiven aufgrund morphologischer und immunhistologischer Parameter. Ophthalmologe. 1995 Aug;92(4):463–468. [PubMed] [Google Scholar]
- Shibagaki I., Shimada Y., Wagata T., Ikenaga M., Imamura M., Ishizaki K. Allelotype analysis of esophageal squamous cell carcinoma. Cancer Res. 1994 Jun 1;54(11):2996–3000. [PubMed] [Google Scholar]
- Simoneau A. R., Spruck C. H., 3rd, Gonzalez-Zulueta M., Gonzalgo M. L., Chan M. F., Tsai Y. C., Dean M., Steven K., Horn T., Jones P. A. Evidence for two tumor suppressor loci associated with proximal chromosome 9p to q and distal chromosome 9q in bladder cancer and the initial screening for GAS1 and PTC mutations. Cancer Res. 1996 Nov 1;56(21):5039–5043. [PubMed] [Google Scholar]
- Snell R. G., MacMillan J. C., Cheadle J. P., Fenton I., Lazarou L. P., Davies P., MacDonald M. E., Gusella J. F., Harper P. S., Shaw D. J. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat Genet. 1993 Aug;4(4):393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Ergazaki M., Arvanitis D., Kiaris H. Microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996 Mar 7;220(1):137–140. doi: 10.1006/bbrc.1996.0370. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Sourvinos G., Kiaris H., Tsamparlakis J. Microsatellite instability and loss of heterozygosity in human pterygia. Br J Ophthalmol. 1997 Jun;81(6):493–496. doi: 10.1136/bjo.81.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash-Bingham C. A., Salazar H., Freed J. J., Greenberg R. E., Tartof K. D. Genomic alterations and instabilities in renal cell carcinomas and their relationship to tumor pathology. Cancer Res. 1995 Dec 15;55(24):6189–6195. [PubMed] [Google Scholar]
- Varinli S., Varinli I., Köksal Erkisi M., Doran F. Human papillomavirus in pterygium. Cent Afr J Med. 1994 Jan;40(1):24–26. [PubMed] [Google Scholar]