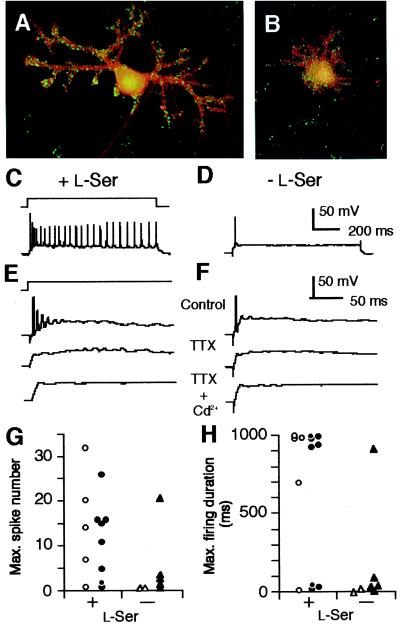
Figure 3.
Effects of exogenous l-Ser on dendritogenesis and excitability of PN. (A and B) Cultured PN maintained for 14 days in the presence (A) or absence (B) of l-Ser (100 μM) were labeled with anti-calbindin D-28K (1:500, mouse monoclonal IgG1, Sigma) and anti-synapsin I (1:200, rabbit polyclonal IgG, Chemicon) antibodies and visualized with Alexa 488 goat anti-mouse (red) and Alexa 546 anti-rabbit IgG conjugates (Molecular Probes, green), and analyzed by a confocal microscopy. (C and F) Voltage responses of PN to current pulse stimuli (schematic in C) recorded in amphotericin-perforated-patch mode. Data taken from PN cultured for 6 days in the presence (C and E) or absence (D and F) of l-Ser (100 μM). The prestimulus membrane potential was set to −65 mV by background current injection. Bath solution includes NBQX (10 μM), d-AP5 (100 μM), and labeled drugs [1 μM tetrodotoxin (TTX) and/or 0.5 mM Cd2+]. Stimulus amplitude: 100 pA in C and D; 300 pA in E and F. (G and H) Maximum number of spikes and maximum duration of firing elicited by a 1-s current pulse stimulus plotted for each cell. Data collected on DIV6–8 from PN identified morphologically (open symbols) or by immunostaining for calbindin D-28K (closed symbols). To determine the maximum number and maximum duration, stimulus amplitudes were incremented in 10-pA steps. The maximum duration was presented as the time between the pulse onset and the last spike. The percentage of neurons exhibiting sustained firing in the l-Ser-treated group (69.2%, n = 13) is significantly higher than that in the untreated group (14.3%, n = 7) (χ2 = 5.94, P = 0.0148, likelihood ratio test). The Erestand input resistance were not different between the l-Ser-treated (−48.9 ± 1.8 mV and 0.33 ± 0.07 GΩ, mean ± SEM) and untreated groups (−44.1 ± 3.6 mV and 0.33 ± 0.07 GΩ).