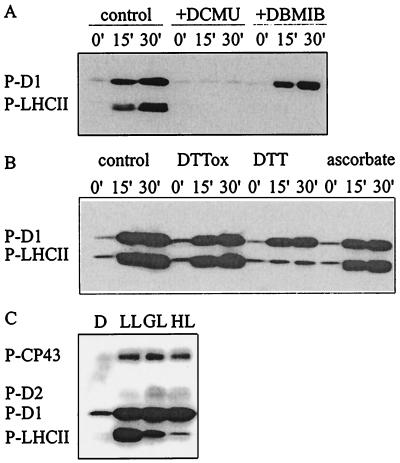
Figure 1.
Modulation of PSII protein phosphorylation by electron transfer inhibitors, reducing agents, and quantity of light. (A and B) Thylakoids isolated from dark-adapted leaves were phosphorylated in vitro for 15 or 30 min in the presence of the following reagents: 20 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) or 10 μM 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB) (A) or 2 mM trans-4,5-dihydroxy-1,2-dithiane (DTTox), 2 mM DTT, or 10 mM ascorbate (B). Immunoblots present the phosphorylation level of the D1 and LHCII proteins. (C) Steady-state phosphorylation level of PSII proteins in vivo. Leaf discs were incubated in darkness (D) or illuminated for 2 h at a photon flux density of 30 (LL), 200 (GL), or 1000 (HL) μmol·m−2·s−1. Phosphorylated PSII proteins were detected by immunoblotting with phosphothreonine antibody. P-CP43, P-D2, P-D1, and P-LHCII, phosphorylated forms of CP43, D2, D1, and LHCII proteins, respectively.