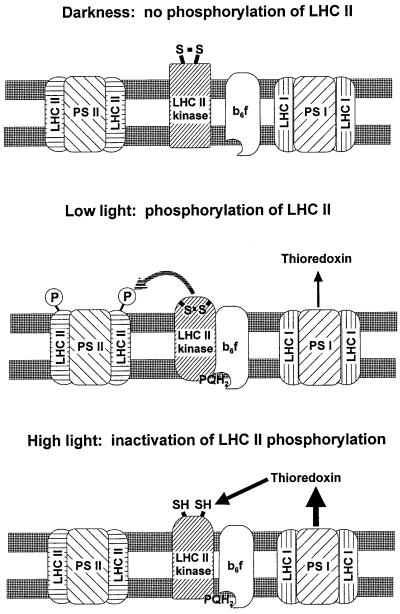
Figure 5.
Model for the regulation of LHCII phosphorylation in vivo. LHCII kinase is inactive in darkness because of the oxidized state of the Qo site in the cytochrome b6/f complex. Light initiates the electron transport in thylakoid membranes, resulting in activation of the LHCII kinase via binding of plastoquinol to the cytochrome b6/f complex. This activation induces a conformational change in the LHCII kinase with a concomitant burial and protection of the target regulatory disulfide bond against reduction. Such an active state prevails in chloroplasts under low light conditions. In high light, the regulatory disulfide bond becomes exposed and reduced via the ferredoxin–thioredoxin system, resulting in an inactive state of LHCII kinase despite the reduced state of the Qo site in cytochrome b6/f complex. The thicknesses of the black arrows indicate the amount of reduced thioredoxins under various light conditions.