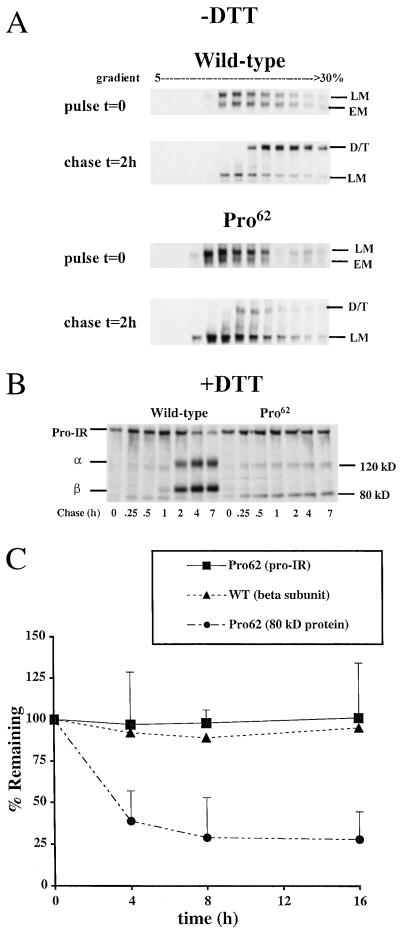
Figure 1.
Misprocessing of mutant IRs. (A) Sucrose density gradient analysis of receptor dimerization. Cells expressing wild-type and Pro62 IR (Pro-IR) were pulse-labeled for 30 min, chased for 2 h, then separated by 5–30% sucrose density gradient. Each gradient fraction was immunoprecipitated with antireceptor antibodies and analyzed by gel electrophoresis. The positions of the folding intermediates are indicated (EM, early monomer; LM, late monomer; D, dimer; T, tetramer). (B) Reducing gel analysis after a 5-min pulse (Pro-IR; α subunit, α; β subunit, β; 120-kDa and 80-kDa bands). (C) Cycloheximide chase. Cells were incubated in cycloheximide, and then cell lysates were processed for immunoblotting with anti-β subunit antibodies at the indicated times. Equivalent amounts of cell lysate protein were analyzed at each time point and the data are represented as the percentage of each subunit present at the beginning of the incubation period (t = 0). Pro-IR, Pro62 proreceptor, 80-kDa protein; WT (β subunit), wild-type IR β subunit. Each point represents data from four separate experiments.