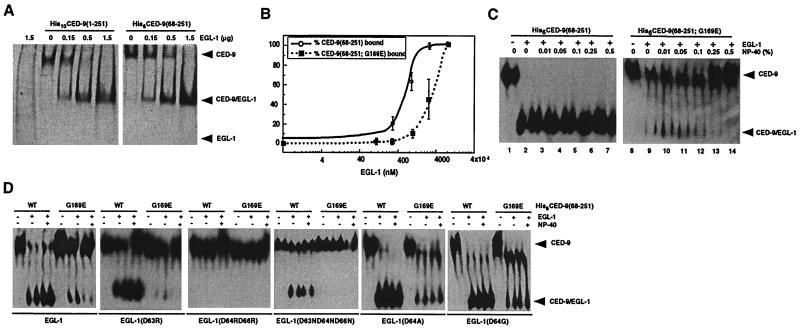
Figure 1.
Characterization of interactions between EGL-1 and CED-9 proteins. (A) CED-9(68–251) is sufficient to bind EGL-1. Purified His10CED-9(1–251) or His6CED-9(68–251) (1.5 μg each) was incubated with the indicated amount of EGL-1 and subjected to native PAGE followed by Coomassie blue staining. (B) The G169E substitution in CED-9 impairs the binding of EGL-1 to CED-9. Purified His6CED-9(68–251) or His6CED-9(68–251; G169E) (500 ng each) was incubated with increasing concentrations of purified EGL-1 and then subjected to native PAGE and Western blotting analysis using anti-His6 antibodies. The amounts of bound and unbound CED-9 were quantified using ImageQuant software (Molecular Dynamics). The percentage of CED-9 in complex with EGL-1 is displayed as a function of the amount of EGL-1 used in the binding reactions. (C) Formation of CED-9(G169E)/EGL-1 complexes but not CED-9/EGL-1 complexes is sensitive to the concentration of the non-ionic detergent Nonidet P-40. Binding reactions were carried out as described (B) using 500 ng of CED-9 and EGL-1 proteins in the presence of the indicated amount of Nonidet P-40. (D) Interactions between CED-9 and mutant EGL-1 proteins. Wild-type or mutant EGL-1 proteins (250 ng each) were incubated with 500 ng of the indicated CED-9 proteins in the presence or absence of 0.25% Nonidet P-40, and the assays were carried out as described (B).