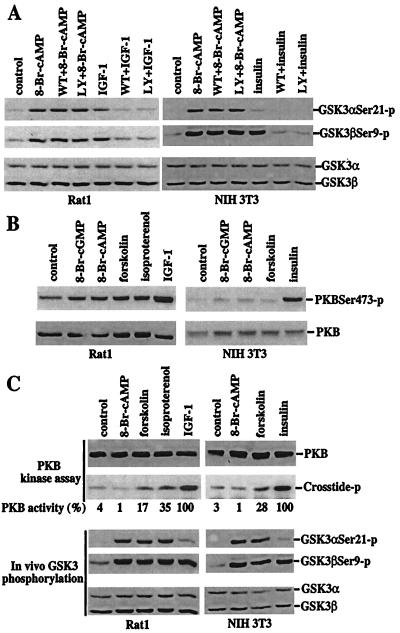
Figure 2.
Dissociation of PKA-mediated phosphorylation and inactivation of GSK-3 from a functional PI3K–PKB signaling pathway. (A) PKA-mediated phosphorylation of GSK-3 is independent of PI3K activity. After 12–24 h of incubation in serum-free medium, Rat1 and NIH 3T3 cells were stimulated with 8-Br-cAMP (2 mM, 30 min), IGF-1 (75 ng/ml, 10 min, Rat1), or insulin (0.1 μM, 10 min, NIH 3T3) in the absence or presence of the PI3K inhibitors, wortmannin (125 nM) or LY294002 (12.5 μM). The cells were preincubated with wortmannin or LY294002 for at least 1 h before addition of 8-Br-cAMP, IGF-1, or insulin. Phosphorylation of GSK-3α at serine 21 and GSK-3β at serine 9 was analyzed by immunoblotting with GSK-3 phospho-specific antibodies as in Fig. 1. (B) Elevation of cAMP levels does not increase phosphorylation of PKB at serine 473. Rat1 and NIH 3T3 cells were starved and stimulated with 8-Br-cGMP, 8-Br-cAMP, forskolin, isoproterenol, IGF, or insulin, as detailed in Fig. 1. PKB phosphorylation levels were analyzed by immunoblotting with a PKB phospho-specific antibody that recognizes PKB phosphorylated at serine 473 (New England Biolabs). Reprobing with a PKB antibody reactive with total PKB (New England Biolabs) shows similar loading among samples. (C) 8-Br-cAMP or elevation of endogenous cAMP levels induces no or limited increases in PKB activity. Rat1 and NIH 3T3 cells were stimulated as in B. PKB activity toward the crosstide–paramyosin substrate was determined as detailed in Materials and Methods. The crosstide–paramyosin substrate phosphorylated at a serine site corresponding to serine 21/9 of GSK-3 is indicated with crosstide-p. The intensities of the crosstide-p bands were quantified by densitometry. The values beneath each lane represent relative intensities (%) with bands induced by IGF-1 in Rat1 cells and by insulin in NIH 3T3 cells defined as 100%. For comparison with PKB activity, a fraction of each lysate was analyzed for levels of GSK-3 phosphorylation at serine 21 and 9 by immunoblotting with GSK-3α and -β phospho-specific antibodies (Bottom). Data shown are representative of three independent experiments.