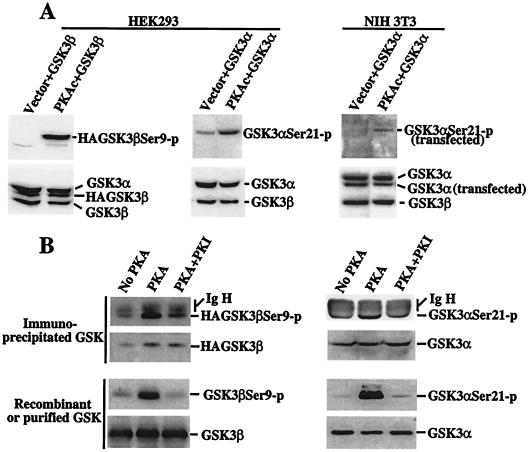
Figure 4.
In vivo and in vitro phosphorylation of GSK-3 by PKA. (A) Transfection of PKAc induces GSK-3 phosphorylation at serine 21 and 9 in intact cells. HEK293 and NIH 3T3 cells were cotransfected with pcDNA3-GSK-3β (labeled as GSK-3β) or pMT2-GSK-3α (GSK-3α) along with an empty vector (Vector) or pcDNA3-PKAc (PKAc). Two days after transfection, cells were starved for 12 h in serum-free medium and lysates prepared. Total cell lysates were analyzed for phosphorylation of transfected and endogenous GSK-3 by immunoblotting with GSK-3α and -β phospho-specific antibodies. Expression of cellular and transfected GSK-3α or -β was determined by immunoblotting with an anti-GSK-3 antibody. The relative locations of cellular and transfected GSK-3α or -β are indicated (Right). (B) PKAc phosphorylates GSK-3 at serine 21 and 9 in vitro. Immunoprecipitated HA-GSK-3β (Top Left), immunoprecipitated GSK-3α (Top Right), recombinant GSK-3β (Bottom Left), and purified GSK-3α (Bottom Right) were used as substrates for PKAc in a kinase reaction. The immunoprecipitates or recombinant/purified GSK-3α or -β were incubated without PKA (No PKA), with PKA (PKA), or with PKA in the presence of the PKA inhibitor PKI (PKA + PKI). Phosphorylation of the GSK-3α or -β substrates by PKA was determined by immunoblotting with GSK-3α and -β phospho-specific antibodies. Levels of GSK-3α or -β substrates were assessed by immunoblotting with an anti-GSK-3 antibody. Bands corresponding to phosphorylated GSK-3α or -β, total GSK-3α or -β, and the Ig heavy chain (IgH) are indicated (Right).