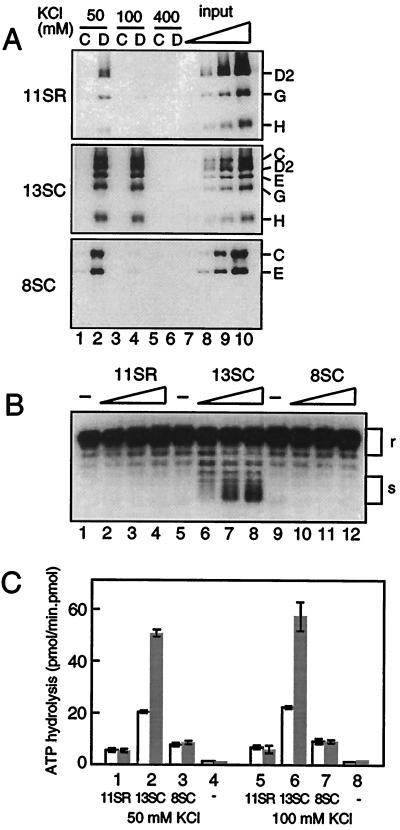
Figure 3.
Biochemical activities of condensin and its subcomplexes. (A) DNA-coupled (lanes 2, 4, and 6) or control (lanes 1, 3, and 5) paramagnetic beads were incubated with the same concentration of purified 11SR (Top), 13SC (Middle), or 8SC (Bottom) in a buffer containing 50 mM KCl (lanes 1 and 2), 100 mM KCl (lanes 3 and 4), or 400 mM KCl (lanes 5 and 6) at 22°C for 1 h. After washing the beads with the same buffer, bound proteins were analyzed by immunoblotting. As standards, 6.3% (lane 7), 12.5% (lane 8), 25% (lane 9), and 50% (lane 10) of input proteins were loaded. (B) Increasing amounts of 11SR, 13SC, or 8SC were incubated with a relaxed circular DNA in the presence of calf thymus topoisomerase I and 1 mM Mg-ATP in a buffer containing 50 mM KCl. DNA was purified, electrophoresed in a 0.7% agarose gel, and visualized by Southern blotting. The positions of relaxed DNA (r) and ladder of supercoiled DNA (s) are indicated. The molar ratios of complex to DNA present in the reaction mixtures were 0 (lanes 1, 5, and 9), 22.5:1 (lanes 2, 6, and 10), 45:1 (lanes 3, 7, and 11), and 90:1 (lanes 4, 8, and 12). (C) An affinity-purified fraction of 11SR (columns 1 and 5), 13SC (columns 2 and 6), 8SC (columns 3 and 7), or buffer alone (columns 4 and 8) was assayed for ATPase activity without DNA (open bar), or with double-stranded DNA (filled bar) at 50 mM KCl or 100 mM KCl. ATP and its hydrolysis product ADP were separated by TLC and quantitated (17). The activities are shown as pmol of hydrolyzed ATP/min⋅pmol of holocomplex or subcomplex.