Abstract
OBJECTIVE—To assess the potential of intravenous Optison, a second generation ultrasound contrast agent, and various ultrasound imaging modes to determine myocardial, kidney, and liver perfusion in normal subjects and patients with left ventricular dysfunction or chronic pulmonary disease together with renal or hepatic dysfunction. METHODS—Five normal subjects and 20 patients underwent grey scale echocardiographic imaging of myocardium, kidney, and liver during 505 intravenous injections of Optison. Images were assessed qualitatively by two independent observers and quantitatively using video densitometry to determine the peak contrast enhancement effect. RESULTS—Qualitative analysis showed that intermittent harmonic imaging was superior to either conventional fundamental or continuous harmonic imaging for all organs. Quantitative analysis showed that the peak change in echocardiographic intensity v baseline during continuous harmonic imaging was 11 units for myocardium (p < 0.03), 7 units for kidney (NS), and 14 units for liver (p < 0.05). During intermittent harmonic imaging the peak change was significantly greater, being 33 units for myocardium (p < 0.0001), 24 units for kidney (p < 0.0002), and 16 units for liver (p < 0.001). CONCLUSIONS—Organ tissue perfusion can be demonstrated following intravenous injection of Optison, particularly when used in combination with intermittent harmonic imaging techniques. This contrast agent is effective in a variety of clinical conditions. Keywords: ultrasound; contrast enhancement; echocardiography; Optison
Full Text
The Full Text of this article is available as a PDF (150.5 KB).
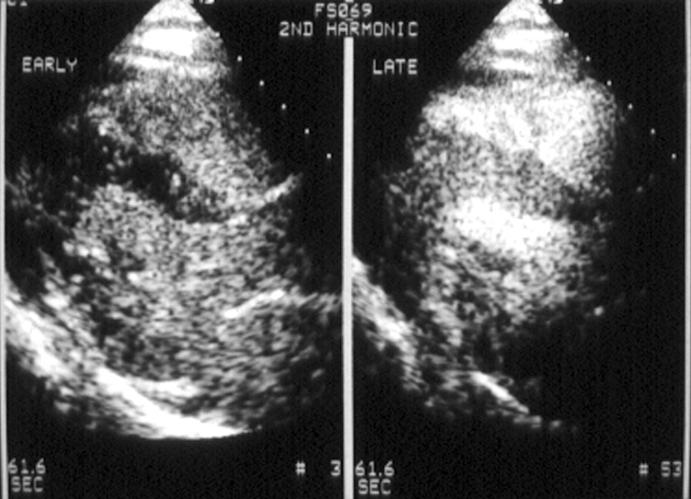
Figure 1 .
Myocardial opacification early (left) and late (right) after a 1 ml injection of Optison during continuous harmonic imaging. Parasternal long axis and apical four chamber views are shown. Early after the injection of Optison, right and left ventricular cavities are opacified but no myocardial opacification is seen. Late after the injection, good myocardial opacification is demonstrated (score of 3 on visual analysis).
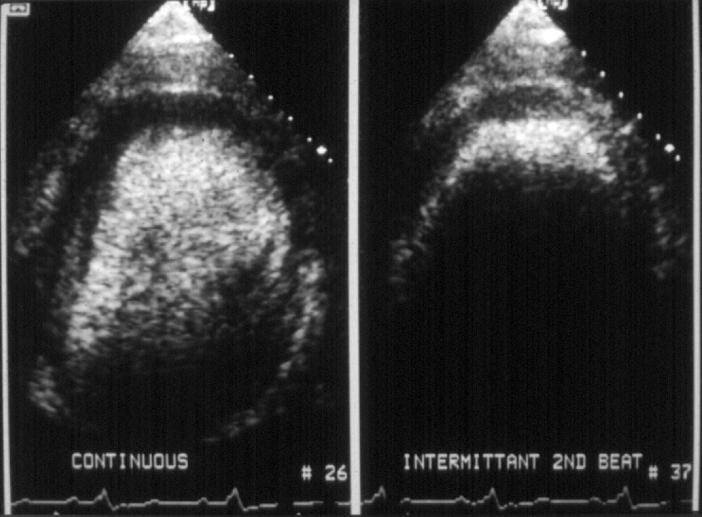
Figure 2 .
Myocardial opacification during continuous (left) and intermittent (right) harmonic imaging every second cardiac cycle following a 1ml injection of Optison. During continuous harmonic imaging, opacification of the left ventricular cavity, septum, and lateral wall is seen, but no opacification at the apex. During intermittent harmonic imaging, less contrast agent is destroyed, which results in visible perfusion at the apex and increased cavity attenuation.
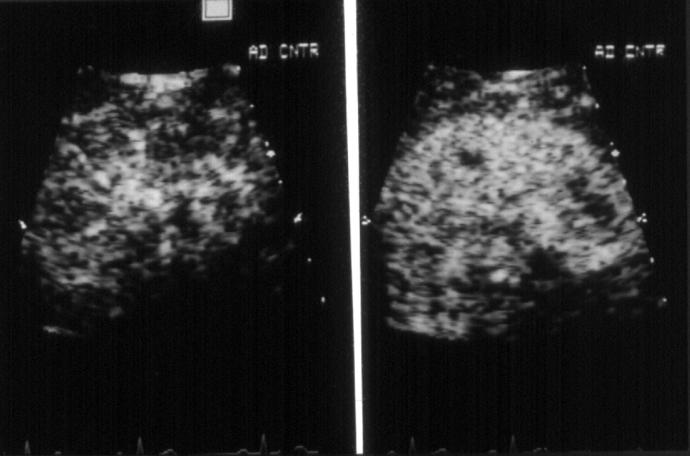
Figure 3 .
Renal opacification during continuous (left) and intermittent (right) harmonic imaging every second cardiac cycle following a 1 ml injection of Optison. During intermittent imaging, less destruction of contrast agent results in increased tissue opacification
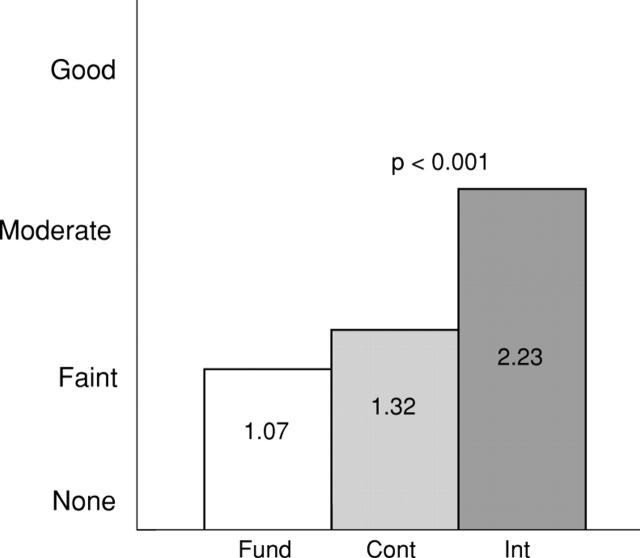
Figure 4 .
Qualitative analysis of peak contrast enhancement effect for all organs at the optimal perfusion volume using a visual scoring system. 0 = none; 1 = faint; 2 = moderate; 3 = good. Cont, continuous harmonic imaging; Fund, fundamental imaging; Int, intermittent harmonic imaging.
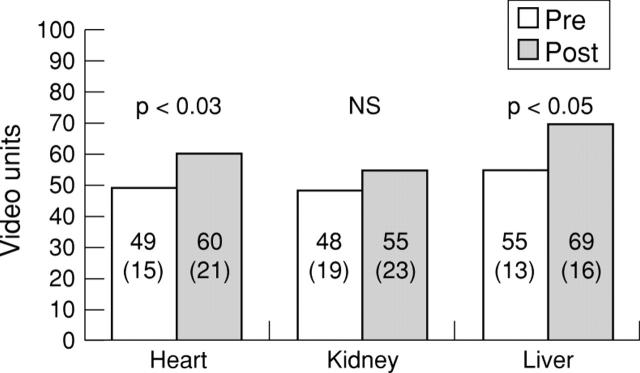
Figure 5 .
Peak video grey scale intensity at baseline and after contrast injection at the optimal perfusion volume for all organs for continuous harmonic imaging. Values are mean (SD).
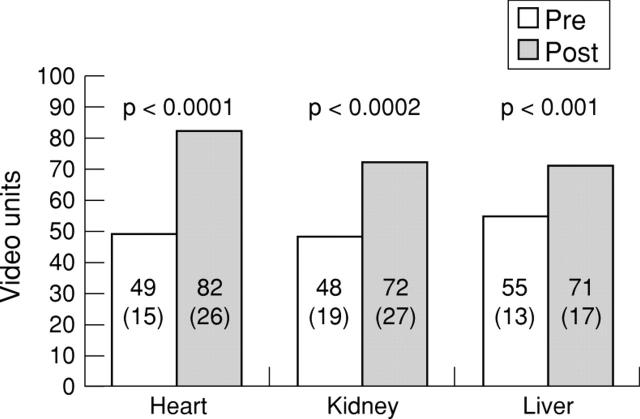
Figure 6 .
Peak video grey scale levels at baseline and following contrast injection at the optimal perfusion volume for all organs during intermittent harmonic imaging every second cardiac cycle. Values are mean (SD).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Cosgrove D. O., Correas J. M., Rallidis L., Nihoyanopoulos P., Patel N. Renal, hepatic, and cardiac enhancement on Doppler and gray-scale sonograms obtained with EchoGen. Acad Radiol. 1996 Aug;3 (Suppl 2):S198–S200. doi: 10.1016/s1076-6332(96)80533-2. [DOI] [PubMed] [Google Scholar]
- Burns P. N. Harmonic imaging with ultrasound contrast agents. Clin Radiol. 1996 Feb;51 (Suppl 1):50–55. [PubMed] [Google Scholar]
- Colon P. J., 3rd, Richards D. R., Moreno C. A., Murgo J. P., Cheirif J. Benefits of reducing the cardiac cycle-triggering frequency of ultrasound imaging to increase myocardial opacification with FSO69 during fundamental and second harmonic imaging. J Am Soc Echocardiogr. 1997 Jul-Aug;10(6):602–607. doi: 10.1016/s0894-7317(97)70022-1. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. Ultrasound contrast enhancement of tumours. Clin Radiol. 1996 Feb;51 (Suppl 1):44–49. [PubMed] [Google Scholar]
- Firschke C., Lindner J. R., Wei K., Goodman N. C., Skyba D. M., Kaul S. Myocardial perfusion imaging in the setting of coronary artery stenosis and acute myocardial infarction using venous injection of a second-generation echocardiographic contrast agent. Circulation. 1997 Aug 5;96(3):959–967. [PubMed] [Google Scholar]
- Forsberg F., Goldberg B. B., Liu J. B., Merton D. A., Rawool N. M. On the feasibility of real-time, in vivo harmonic imaging with proteinaceous microspheres. J Ultrasound Med. 1996 Dec;15(12):853–862. doi: 10.7863/jum.1996.15.12.853. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Echo-enhancing agents in the renal tract. Clin Radiol. 1996 Feb;51 (Suppl 1):40–43. [PubMed] [Google Scholar]
- Kaul S. New developments in ultrasound systems for contrast echocardiography. Clin Cardiol. 1997 Oct;20(10 Suppl 1):I27–I30. doi: 10.1002/clc.4960201307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S., Senior R., Dittrich H., Raval U., Khattar R., Lahiri A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation. 1997 Aug 5;96(3):785–792. [PubMed] [Google Scholar]
- Kono Y., Moriyasu F., Nada T., Suginoshita Y., Matsumura T., Kobayashi K., Nakamura T., Chiba T. Gray scale second harmonic imaging of the liver: a preliminary animal study. Ultrasound Med Biol. 1997;23(5):719–726. doi: 10.1016/s0301-5629(97)00007-0. [DOI] [PubMed] [Google Scholar]
- Leen E., McArdle C. S. Ultrasound contrast agents in liver imaging. Clin Radiol. 1996 Feb;51 (Suppl 1):35–39. [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Mulvagh S. L., Foley D. A., Aeschbacher B. C., Klarich K. K., Seward J. B. Second harmonic imaging of an intravenously administered echocardiographic contrast agent: Visualization of coronary arteries and measurement of coronary blood flow. J Am Coll Cardiol. 1996 May;27(6):1519–1525. doi: 10.1016/0735-1097(95)00619-2. [DOI] [PubMed] [Google Scholar]
- Skyba D. M., Camarano G., Goodman N. C., Price R. J., Skalak T. C., Kaul S. Hemodynamic characteristics, myocardial kinetics and microvascular rheology of FS-069, a second-generation echocardiographic contrast agent capable of producing myocardial opacification from a venous injection. J Am Coll Cardiol. 1996 Nov 1;28(5):1292–1300. doi: 10.1016/S0735-1097(96)00328-2. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Jr AHA president's letter. Circulation. 1995 Jul 1;92(1):1–1. [PubMed] [Google Scholar]
- Villarraga H. R., Foley D. A., Aeschbacher B. C., Klarich K. W., Mulvagh S. L. Destruction of contrast microbubbles during ultrasound imaging at conventional power output. J Am Soc Echocardiogr. 1997 Oct;10(8):783–791. doi: 10.1016/s0894-7317(97)70036-1. [DOI] [PubMed] [Google Scholar]
- Wei K., Skyba D. M., Firschke C., Jayaweera A. R., Lindner J. R., Kaul S. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol. 1997 Apr;29(5):1081–1088. doi: 10.1016/s0735-1097(97)00029-6. [DOI] [PubMed] [Google Scholar]