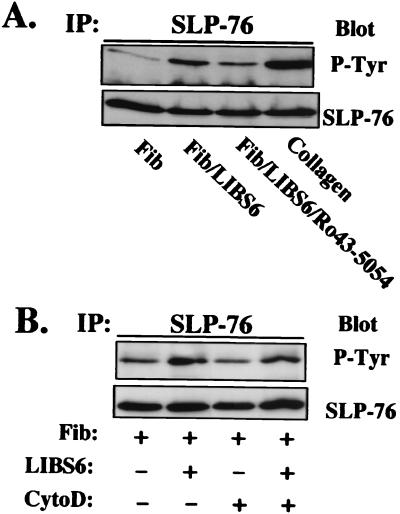
Figure 1.
SLP-76 is tyrosine phosphorylated in response to fibrinogen binding to αIIbβ3 independent of actin polymerization. (A) Platelets were incubated with 250 μg/ml human fibrinogen (lane 1), fibrinogen plus 150 μg/ml LIBS-6 Fab (lane 2), fibrinogen plus LIBS-6 Fab plus 10 μM Ro43–5054 (lane 3), or 5 μg/ml collagen (lane 4). Platelets were lysed, immunoprecipitated with anti-human SLP-76, and analyzed by SDS/PAGE and immunoblot for phosphotyrosine. The top blot was stripped and reprobed with anti-human SLP-76 to demonstrate equal amounts of immunoprecipitated SLP-76 in each lane. Similar results were obtained in three separate experiments. (B) Washed human platelets were incubated for 10 min at room temperature in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of 10 μM cytochalasin D and then stimulated with 250 μg/ml fibrinogen alone (lanes 1 and 3), or 150 μg/ml LIBS-6 Fab plus 250 μg/ml fibrinogen (lanes 2 and 4).