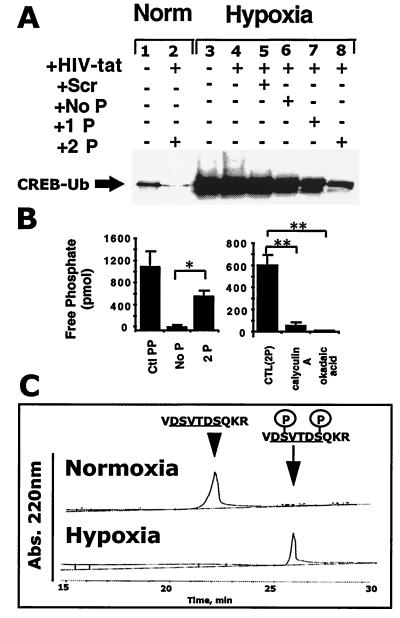
Figure 3.
Synthetic CREB targeting phosphopeptide sequences inhibit CREB ubiquitination in a phosphorylation-dependent manner. (A) Baseline level of CREB ubiquitination is detectable in T84 cells (Lane 1). Lane 2 represents normoxic CREB in the presence of the double-phosphorylated CREB-targeting phosphopeptide. Lane 3 represents hypoxic CREB in the absence of peptide treatment, and lanes 4–8 (left to right) represent CREB from hypoxia-treated cells in the presence of the HIV-tat peptide alone, HIV-tat with scrambled peptide, HIV-tat with the unphosphorylated CREB-targeting peptide, HIV-tat with the single-phosphorylated CREB-targeting peptide, and HIV-tat with the double-phosphorylated CREB-targeting peptide, respectively. Data are representative of two experiments. (B) The CREB targeting sequence is a substrate for PP1 dephosphorylation. (Left) Coincubation of the phosphorylated form of the CREB-targeting sequence (2P) with purified PP1 results in the liberation of free phosphate when compared with the unphosphorylated control (Ctl; No P; n = 6, P < 0.05). A threonine phosphopeptide (Ctl PP) served as a positive control. (Right) The phosphatase inhibitors okadaic acid (4 μM) and calyculin A (400 nM) abolished PP1-mediated dephosphorylation of the phosphorylated targeting sequence of CREB (n = 6, P < 0.05). (C) Hypoxia decreases phosphopeptide dephosphorylation. Coincubation of the double-phosphorylated CREB-targeting sequence (2P) with normoxic T84 (Upper) but not hypoxic (Lower) lysates results in peptide dephosphorylation as measured by HPLC (representative tracings of two experiments). Abs., absorbance.