Abstract
Background/Aim—Human papillomaviruses (HPVs) are important, but not sufficient, for the development of cervical cancer. All three human ß-herpesviruses—cytomegalovirus (CMV) and human herpesviruses (HHV) types 6 and 7—have been detected in the cervix. In addition, CMV and HHV-6 can interact with HPVs in vivo. This study examined the possible role of ß-herpesviruses in cervical cancer development.
Methods—HPV, CMV, HHV-6, and HHV-7 were detected by the polymerase chain reaction using cervical scrapes taken at colposcopy from 388 women. HPV types were identified using restriction fragment length polymorphisms. Colposcopy guided biopsies were taken from abnormal areas, and the histological findings were regarded as the final diagnoses. The associations between herpesvirus infection and the degree of cervical lesion were analysed with respect to HPV status.
Results—Of the 388 women, 51.8% had a normal cervix, 14.4% had cervical intraepithelial neoplasia grade 1 (CIN1), 8.2% had CIN2, 19.3% had CIN3, and 6.2% had invasive carcinoma. Overall, the positive rates for high, intermediate, and low risk HPVs were 18.8%, 21.4%, and 5.2%, respectively. Fifteen patients harboured HPVs for which the genotype could not be identified. Positive rates for CMV, HHV-6, and HHV-7 were 9.5%, 3.6%, and 3.4%, respectively. HPV positive patients carried a higher risk for high grade lesions (CIN2/3 or carcinoma) (odds ratio (OR), 5.24; 95% confidence interval (CI), 3.19 to 8.62; χ2 = 51.79; p < 0.001), whereas those positive for CMV, HHV-6, or HHV-7 did not. Thirteen of 131 patients with high grade lesions had HPV/herpesvirus coinfections, but no association with the cervical lesion was noted. Furthermore, positive rates for herpesviruses among HPV negative, high/intermediate risk HPV negative, and high risk HPV negative subgroups were similarly low and without a significant association.
Conclusions—The ubiquitous nature of herpesviruses may pose difficulty in elucidating their pathogenic role. These results indicate that CMV, HHV-6, and HHV-7 are bystanders rather than cofactors in the oncogenesis of cervical cancer.
Key Words: human papillomavirus • human herpesvirus 6 • human herpesvirus 7
Full Text
The Full Text of this article is available as a PDF (194.7 KB).
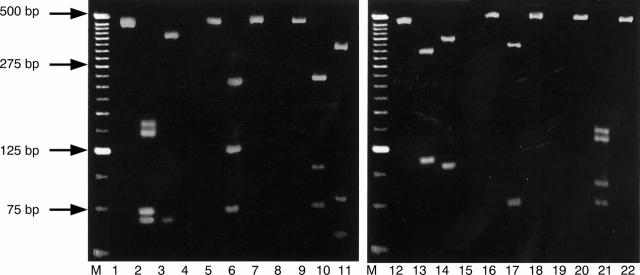
Figure 1 Restriction fragment length patterns of representative human papillomavirus (HPV) genotypes obtained by endonuclease digestion of L1 open reading frame (ORF) PCR products. Lane M: 25 bp ladder marker. Lanes 1–3: HPV6 not restricted, restricted with RsaI, and restricted with DdeI, respectively. Lane 4: blank. Lanes 5–7: HPV11 not restricted, restricted with RsaI, and restricted with DdeI, respectively. Lane 8: blank. Lanes 9–11: HPV33 not restricted, restricted with RsaI, and restricted with DdeI, respectively. Lanes 12–14: HPV58 not restricted, restricted with RsaI, and restricted with DdeI, respectively. Lane 15: blank. Lanes 16–18: HPV16 not restricted, restricted with RsaI, and restricted with DdeI, respectively. Lane 19: blank. Lanes 20–22: HPV18 not restricted, restricted with RsaI, and restricted with DdeI, respectively.
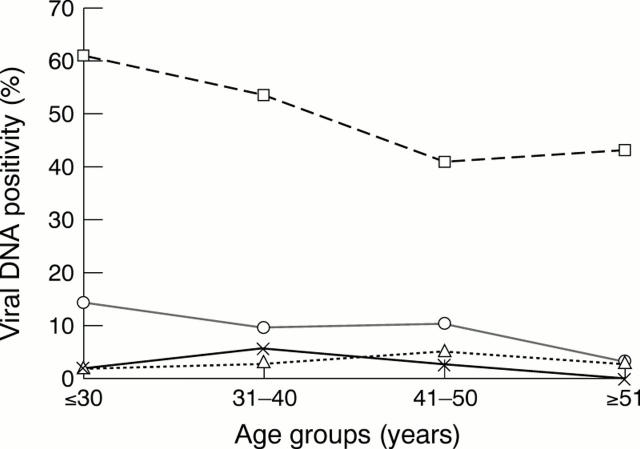
Figure 2 Prevalence of human papillomavirus (HPV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), and HHV-7 in the cervix of 388 women with abnormal cervical cytologies by age groups. Numbers of patients tested in each age group: ≤ 30 years, 56 patients; 31–40 years, 159 patients; 41–50 years, 115 patients; > 51 years, 58 patients. Squares, HPV; circles, CMV; triangles, HHV-6; crosses, HHV-7.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H. M., Ting Y., Greer C. E., Chambers J. C., Tashiro C. J., Chimera J., Reingold A., Manos M. M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991 Jan 23;265(4):472–477. [PubMed] [Google Scholar]
- Bernard H. U., Chan S. Y., Manos M. M., Ong C. K., Villa L. L., Delius H., Peyton C. L., Bauer H. M., Wheeler C. M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994 Nov;170(5):1077–1085. doi: 10.1093/infdis/170.5.1077. [DOI] [PubMed] [Google Scholar]
- Berneman Z. N., Ablashi D. V., Li G., Eger-Fletcher M., Reitz M. S., Jr, Hung C. L., Brus I., Komaroff A. L., Gallo R. C. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I., AbuBakar S., Deng C. Z., Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991 Mar;65(3):1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock K. E., Berry G., Mock P. A., MacLennan R., Truswell A. S., Brinton L. A. Nutrients in diet and plasma and risk of in situ cervical cancer. J Natl Cancer Inst. 1988 Jun 15;80(8):580–585. doi: 10.1093/jnci/80.8.580. [DOI] [PubMed] [Google Scholar]
- Chan P. K., Li W. H., Chan M. Y., Ma W. L., Cheung J. L., Cheng A. F. High prevalence of human papillomavirus type 58 in Chinese women with cervical cancer and precancerous lesions. J Med Virol. 1999 Oct;59(2):232–238. [PubMed] [Google Scholar]
- Chen M., Popescu N., Woodworth C., Berneman Z., Corbellino M., Lusso P., Ablashi D. V., DiPaolo J. A. Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. J Virol. 1994 Feb;68(2):1173–1178. doi: 10.1128/jvi.68.2.1173-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. C., Byrne J. C., Rabson A. S. Human cytomegalovirus (HCMV) enhances bovine papilloma virus (BPV) transformation in vitro. J Med Virol. 1987 Oct;23(2):157–164. doi: 10.1002/jmv.1890230208. [DOI] [PubMed] [Google Scholar]
- Gopal M. R., Thomson B. J., Fox J., Tedder R. S., Honess R. W. Detection by PCR of HHV-6 and EBV DNA in blood and oropharynx of healthy adults and HIV-seropositives. Lancet. 1990 Jun 30;335(8705):1598–1599. doi: 10.1016/0140-6736(90)91433-b. [DOI] [PubMed] [Google Scholar]
- Gradilone A., Vercillo R., Napolitano M., Cardinali G., Gazzaniga P., Silvestri I., Gandini O., Tomao S., Aglianò A. M. Prevalence of human papillomavirus, cytomegalovirus, and Epstein-Barr virus in the cervix of healthy women. J Med Virol. 1996 Sep;50(1):1–4. doi: 10.1002/(SICI)1096-9071(199609)50:1<1::AID-JMV1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Han C. P., Tsao Y. P., Sun C. A., Ng H. T., Chen S. L. Human papillomavirus, cytomegalovirus and herpes simplex virus infections for cervical cancer in Taiwan. Cancer Lett. 1997 Dec 9;120(2):217–221. doi: 10.1016/s0304-3835(97)00312-1. [DOI] [PubMed] [Google Scholar]
- Herrington C. S. Human papillomaviruses and cervical neoplasia. I. Classification, virology, pathology, and epidemiology. J Clin Pathol. 1994 Dec;47(12):1066–1072. doi: 10.1136/jcp.47.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington C. S. Human papillomaviruses and cervical neoplasia. II. Interaction of HPV with other factors. J Clin Pathol. 1995 Jan;48(1):1–6. doi: 10.1136/jcp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska S., Dabrowski J., Jakubowicz K. Epidermodysplasia verruciformis as a model in studies on the role of papovaviruses in oncogenesis. Cancer Res. 1972 Mar;32(3):583–589. [PubMed] [Google Scholar]
- Jarrett W. F., Murphy J., O'Neil B. W., Laird H. M. Virus-induced papillomas of the alimentary tract of cattle. Int J Cancer. 1978 Sep 15;22(3):323–328. doi: 10.1002/ijc.2910220316. [DOI] [PubMed] [Google Scholar]
- Khan G., Kangro H. O., Coates P. J., Heath R. B. Inhibitory effects of urine on the polymerase chain reaction for cytomegalovirus DNA. J Clin Pathol. 1991 May;44(5):360–365. doi: 10.1136/jcp.44.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer S. K., de Villiers E. M., Cağlayan H., Svare E., Haugaard B. J., Engholm G., Christensen R. B., Møller K. A., Poll P., Jensen H. Human papillomavirus, herpes simplex virus and other potential risk factors for cervical cancer in a high-risk area (Greenland) and a low-risk area (Denmark)--a second look. Br J Cancer. 1993 Apr;67(4):830–837. doi: 10.1038/bjc.1993.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa M., Koumantakis E., Ergazaki M., Tsatsanis C., Spandidos D. A. Association of herpesvirus infection with the development of genital cancer. Int J Cancer. 1995 Sep 27;63(1):58–62. doi: 10.1002/ijc.2910630112. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. In vitro amplification of hepatitis B virus sequences from liver tumour DNA and from paraffin wax embedded tissues using the polymerase chain reaction. J Clin Pathol. 1989 Aug;42(8):840–846. doi: 10.1136/jcp.42.8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Oishi H., Hayashi K., Nonogaki M., Tanaka K., Yamanishi K. Human herpesviruses 6 and 7 in cervixes of pregnant women. J Clin Microbiol. 1995 Jul;33(7):1968–1970. doi: 10.1128/jcm.33.7.1968-1970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Black J. B., Yamamoto M. Human herpesvirus 6: the virus and the search for its role as a human pathogen. Adv Virus Res. 1992;41:1–52. doi: 10.1016/s0065-3527(08)60034-2. [DOI] [PubMed] [Google Scholar]
- Razzaque A., Williams O., Wang J., Rhim J. S. Neoplastic transformation of immortalized human epidermal keratinocytes by two HHV-6 DNA clones. Virology. 1993 Jul;195(1):113–120. doi: 10.1006/viro.1993.1351. [DOI] [PubMed] [Google Scholar]
- Romano N., Romano F. M., Viviano E., Vitale F., Villafrate M. R., Perna A. M., Bonura F., Guttadauro R. Rare association of human herpesvirus 6 DNA with human papillomavirus DNA in cervical smears of women with normal and abnormal cytologies. J Clin Microbiol. 1996 Jun;34(6):1589–1591. doi: 10.1128/jcm.34.6.1589-1591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada E., Yasukawa M., Ito C., Takeda A., Shiosaka T., Tanioka H., Fujita S. Detection of human herpesvirus 6 and human herpesvirus 7 in the submandibular gland, parotid gland, and lip salivary gland by PCR. J Clin Microbiol. 1996 Sep;34(9):2320–2321. doi: 10.1128/jcm.34.9.2320-2321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmauz R., Okong P., de Villiers E. M., Dennin R., Brade L., Lwanga S. K., Owor R. Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. Int J Cancer. 1989 May 15;43(5):805–809. doi: 10.1002/ijc.2910430511. [DOI] [PubMed] [Google Scholar]
- Shen C. Y., Ho M. S., Chang S. F., Yen M. S., Ng H. T., Huang E. S., Wu C. W. High rate of concurrent genital infections with human cytomegalovirus and human papillomaviruses in cervical cancer patients. J Infect Dis. 1993 Aug;168(2):449–452. doi: 10.1093/infdis/168.2.449. [DOI] [PubMed] [Google Scholar]
- Sood A. K. Cigarette smoking and cervical cancer: meta-analysis and critical review of recent studies. Am J Prev Med. 1991 Jul-Aug;7(4):208–213. [PubMed] [Google Scholar]
- Thompson C. H., Rose B. R., Elliott P. M. Cytomegalovirus and cervical cancer: failure to detect a direct association or an interaction with human papillomaviruses. Gynecol Oncol. 1994 Jul;54(1):40–46. doi: 10.1006/gyno.1994.1163. [DOI] [PubMed] [Google Scholar]
- Thompson J., Choudhury S., Kashanchi F., Doniger J., Berneman Z., Frenkel N., Rosenthal L. J. A transforming fragment within the direct repeat region of human herpesvirus type 6 that transactivates HIV-1. Oncogene. 1994 Apr;9(4):1167–1175. [PubMed] [Google Scholar]
- Vessey M., Grice D. Carcinoma of the cervix and oral contraceptives: epidemiological studies. Biomed Pharmacother. 1989;43(3):157–160. doi: 10.1016/0753-3322(89)90208-4. [DOI] [PubMed] [Google Scholar]
- Wang F. Z., Dahl H., Linde A., Brytting M., Ehrnst A., Ljungman P. Lymphotropic herpesviruses in allogeneic bone marrow transplantation. Blood. 1996 Nov 1;88(9):3615–3620. [PubMed] [Google Scholar]
- Wang H., Chen M., Berneman Z. N., Delgado G., DiPaolo J. A. Detection of human herpesvirus-6 in paraffin-embedded tissue of cervical cancer by polymerase chain reaction. J Virol Methods. 1994 May;47(3):297–305. doi: 10.1016/0166-0934(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Yadav M., Arivananthan M., Kumar S. HHV-6 antigen and viral DNA detected in cervical cells from archived tissue using histochemical staining and hybridization. Clin Diagn Virol. 1996 Oct;7(1):23–33. doi: 10.1016/s0928-0197(96)00250-4. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1–30. doi: 10.1007/978-3-642-66800-5_1. [DOI] [PubMed] [Google Scholar]