Abstract
Aim—Biopsies of the gastric antrum were reviewed over a period of 10 years to determine the prevalence of Helicobacter heilmannii in symptomatic subjects from this geographical area and to relate its presence to distinctive histopathological and immunohistochemical features.
Methods—Biopsies from 7926 symptomatic patients were reviewed. Ten serial sections were stained with haematoxylin and eosin for conventional histology. Another 10 sections were stained with the Gram method for spiral bacteria. When H heilmannii was suspected, 10 additional serial sections were stained with methylene blue to obtain homogeneous colouring. An equal number of sections from patients affected by isolated H heilmannii or H pylori gastritis were analysed by immunohistochemistry to evaluate lymphoid aggregate/mucosal lymphocyte clonality (CD20 and CD3) and tumour necrosis factor alpha (TNF-α) in stromal cells.
Results—The prevalence of H heilmannii was 0.1% (eight of 7926), whereas H pylori was present in 60.7% of patients (4813 of 7926). In two of the eight H heilmannii positive patients both helicobacters were found. In all subjects infected by H heilmannii only, distinctive histology (lymphocyte exudation into gastric foveolae) was seen. Lymphoid aggregates, chronic mucosal inflammation with patchy activity, and the absence of epithelial mucus depletion were regular features of H heilmannii gastritis. Immunohistochemistry did not reveal different lymphocyte clonal patterns between H pylori and H heilmannii gastritis: CD20 positive cells were predominant in the centre of aggregates and mucosal infiltrates, whereas CD3 positive cells were prevalent at the periphery of follicles. Only H pylori gastritis showed a significant increase in TNF-α positive stromal cells.
Conclusion—These data suggest that an unusual lymphocyte reaction, with the tendency to invade the foveolar lumen, is a distinctive histopathological aspect of H heilmannii chronic gastritis, although further studies in a larger series are necessary to confirm this fact. Nevertheless, lymphocyte clones do not differ qualitatively from those found in H pylori infection. Moreover, compared with H heilmannii, H pylori provokes a more intense release of TNF-α, suggesting that different inflammatory responses exist to these two organisms.
Key Words: Helicobacter heilmannii • Helicobacter pylori • tumour necrosis factor α
Full Text
The Full Text of this article is available as a PDF (201.8 KB).
Figure 1 (A, B) Helicobacter heilmannii in gastric antral foveola (methylene blue stain): "corrugated cigar" and "ball-like" aspects (original magnification, x1000).
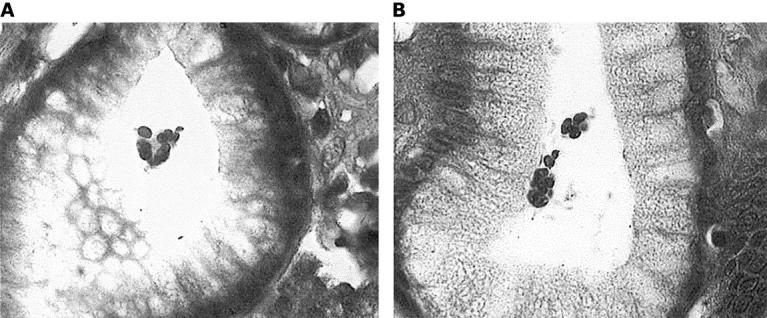
Figure 2 (A, B) Lymphocyte exudation into the lumen of gastric crypts in patient with Helicobacter heilmannii gastritis (original magnification, x1000).
Figure 3 (A, B) Immunohistochemical staining of tumour necrosis factor α (TNF-α) in stromal cells of gastric antrum from a Helicobacter pylori and H heilmannii positive patient. The cytoplasm of positive cells is stained red by aminoethylcarbazole and strong positivity is seen (original magnification, x400).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen L. P., Boye K., Blom J., Holck S., Norgaard A., Elsborg L. Characterization of a culturable "Gastrospirillum hominis" (Helicobacter heilmannii) strain isolated from human gastric mucosa. J Clin Microbiol. 1999 Apr;37(4):1069–1076. doi: 10.1128/jcm.37.4.1069-1076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert F. J., D'Haens G. R., Peeters M., Hiele M. I., Schaible T. F., Shealy D., Geboes K., Rutgeerts P. J. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999 Jan;116(1):22–28. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- Dent J. C., McNulty C. A., Uff J. C., Wilkinson S. P., Gear M. W. Spiral organisms in the gastric antrum. Lancet. 1987 Jul 11;2(8550):96–96. doi: 10.1016/s0140-6736(87)92754-1. [DOI] [PubMed] [Google Scholar]
- Dieterich C., Wiesel P., Neiger R., Blum A., Corthésy-Theulaz I. Presence of multiple "Helicobacter heilmannii" strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998 May;36(5):1366–1370. doi: 10.1128/jcm.36.5.1366-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Quaranta S. Spiral shaped bacteria in gastric mucosa. J Clin Pathol. 1990 Feb;43(2):173–173. doi: 10.1136/jcp.43.2.173-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschini M. P., Pieri F., Cerasoli S., Accardo P., Formica G., Biasiucci A., Donzelli C., Villanacci V. Helicobacter heilmannii: studio anatomo-clinico di 14 nuovi casi. Pathologica. 1999 Feb;91(1):18–24. [PubMed] [Google Scholar]
- Goteri G., Ranaldi R., Rezai B., Baccarini M. G., Bearzi I. Synchronous mucosa-associated lymphoid tissue lymphoma and adenocarcinoma of the stomach. Am J Surg Pathol. 1997 May;21(5):505–509. doi: 10.1097/00000478-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Heilmann K. L., Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991 Feb;32(2):137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilzenrat N., Lamoureux E., Weintrub I., Alpert E., Lichter M., Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995 Dec;119(12):1149–1153. [PubMed] [Google Scholar]
- Holck S., Ingeholm P., Blom J., Nørgaard A., Elsborg L., Adamsen S., Andersen L. P. The histopathology of human gastric mucosa inhabited by Helicobacter heilmannii-like (Gastrospirillum hominis) organisms, including the first culturable case. APMIS. 1997 Oct;105(10):746–756. doi: 10.1111/j.1699-0463.1997.tb05080.x. [DOI] [PubMed] [Google Scholar]
- Ierardi E., Monno R., Mongelli A., Allegretta L., Milone E., Rizzi S., Panza P., Coppolecchia P., Francavilla A. Gastrospirillum Hominis associated chronic active gastritis: the first report from Italy. Ital J Gastroenterol. 1991 Feb;23(2):86–87. [PubMed] [Google Scholar]
- Kim J. M., Kim J. S., Jung H. C., Song I. S., Kim C. Y. Apoptosis of human gastric epithelial cells via caspase-3 activation in response to Helicobacter pylori infection: possible involvement of neutrophils through tumor necrosis factor alpha and soluble Fas ligands. Scand J Gastroenterol. 2000 Jan;35(1):40–48. doi: 10.1080/003655200750024515. [DOI] [PubMed] [Google Scholar]
- Mattapallil J. J., Dandekar S., Canfield D. R., Solnick J. V. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000 Feb;118(2):307–315. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- Meining A., Kroher G., Stolte M. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand J Gastroenterol. 1998 Aug;33(8):795–798. doi: 10.1080/00365529850171422. [DOI] [PubMed] [Google Scholar]
- Monno R., Ierardi E., Valenza M. A., Campanale A., Francavilla A., Fumarola L. Gastrospirillum hominis and human chronic gastritis. New Microbiol. 1995 Oct;18(4):441–444. [PubMed] [Google Scholar]
- Norris C. R., Marks S. L., Eaton K. A., Torabian S. Z., Munn R. J., Solnick J. V. Healthy cats are commonly colonized with "Helicobacter heilmannii" that is associated with minimal gastritis. J Clin Microbiol. 1999 Jan;37(1):189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regimbeau C., Karsenti D., Durand V., D'Alteroche L., Copie-Bergman C., Metman E. H., Machet M. C. Lymphome gastrique de bas grade du MALT et Helicobacter heilmannii (Gastrospirillum hominis). Gastroenterol Clin Biol. 1998 Aug-Sep;22(8-9):720–723. [PubMed] [Google Scholar]
- Saxena A., Moshynska O., Kanthan R., Bhutani M., Maksymiuk A. W., Lukie B. E. Distinct B-cell clonal bands in Helicobacter pylori gastritis with lymphoid hyperplasia. J Pathol. 2000 Jan;190(1):47–54. doi: 10.1002/(SICI)1096-9896(200001)190:1<47::AID-PATH506>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Solnick J. V., O'Rourke J., Lee A., Paster B. J., Dewhirst F. E., Tompkins L. S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993 Aug;168(2):379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- Stolte M., Kroher G., Meining A., Morgner A., Bayerdörffer E., Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997 Jan;32(1):28–33. doi: 10.3109/00365529709025059. [DOI] [PubMed] [Google Scholar]
- Stolte M., Wellens E., Bethke B., Ritter M., Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994 Dec;29(12):1061–1064. doi: 10.3109/00365529409094888. [DOI] [PubMed] [Google Scholar]
- Tee W., Lambert J. R., Dwyer B. Cytotoxin production by Helicobacter pylori from patients with upper gastrointestinal tract diseases. J Clin Microbiol. 1995 May;33(5):1203–1205. doi: 10.1128/jcm.33.5.1203-1205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]




