Abstract
Aims—Although frequent reduction or loss of DCC (deleted in colorectal carcinomas) has been demonstrated in gliomas, the association with cell kinetics and survival is still unclear.
Methods—A total of 119 astrocytomas, comprising 39 grade IV, 36 grade III, and 44 low grade tumours, were immunohistochemically investigated, along with 26 normal adult brain samples and two fetal brains. The results were compared with p53 abnormalities, Ki-67 labelling index (LI), mitotic index (MI), apoptotic index (AI), and survival.
Results—In normal adult and fetal brain tissues, DCC expression was detected in mature and terminally differentiated neuronal cells but not glial elements. In astrocytomas, whereas DCC expression was still clearly shown with low grade malignancy, DCC scores were significantly decreased in high histological grade malignancy, along with an increase in cell kinetics determined by AI, MI, and Ki-67 LI values. In addition, p53 LI values were significantly increased, although a direct link between DCC scores and p53 LI values was not evident. Univariate analysis revealed that high DCC scores and low p53 LI values were closely related to a favourable outcome for astrocytoma, although only the AI was an independent prognostic factor.
Conclusions—The loss of DCC expression may be closely related to changes in cell kinetics and tumour phenotype in astrocytomas, independent of p53 abnormalities.
Key Words: astrocytoma • deleted in colorectal carcinoma gene • p53 • cell kinetics
Full Text
The Full Text of this article is available as a PDF (300.8 KB).
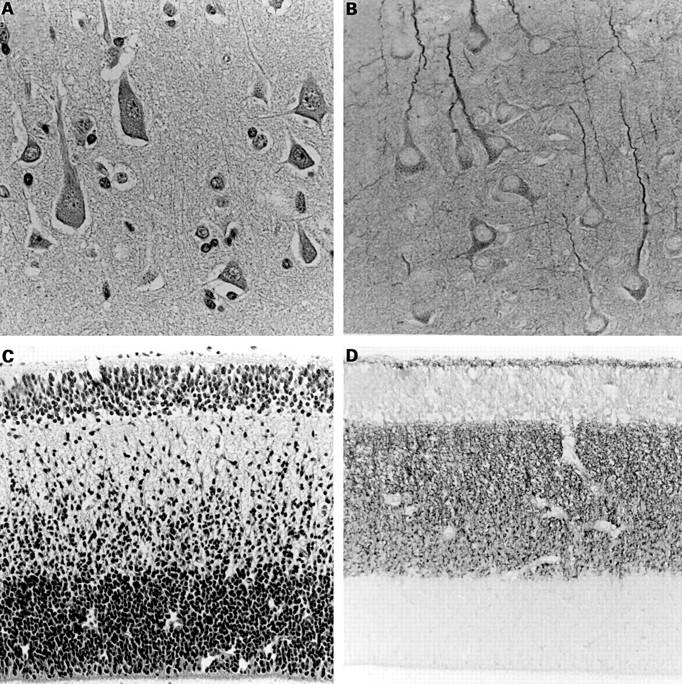
Figure 1 (A, C) Haematoxylin and eosin staining and (B, D) DCC (deleted in colorectal carcinoma) immunoreactivity in adult (A, B) and fetal (C, D) normal brain tissue. (B) DCC immunopositivity is evident in axons and nerve cell bodies, but not in glial cells. (D) Note that neuronal and glial precursor cells in the ventricular and subventricular zone (lower lamina) are negative. Original magnification (A, B), x400; (C, D) x200.
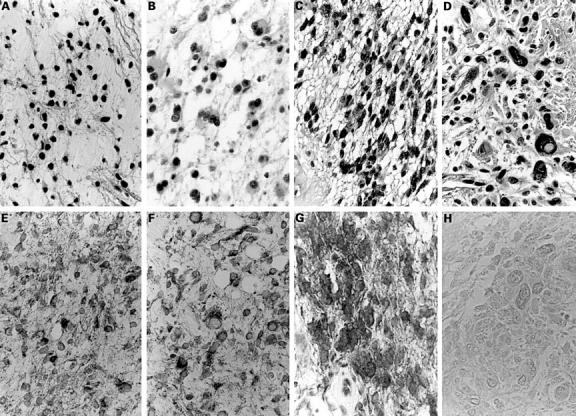
Figure 2 Haematoxylin and eosin staining (A, B, C, D) and DCC (deleted in colorectal carcinoma) immnoreactivity (E, F, G, H) in low grade astrocytomas (A, E: fibrillary subtype; B, F: gemistocytic subtype), an anaplastic astrocytoma (C, G), and a glioblastoma (D, H). Note strong intensities (E, F, G), and absence of staining (H). Original magnification, x400.
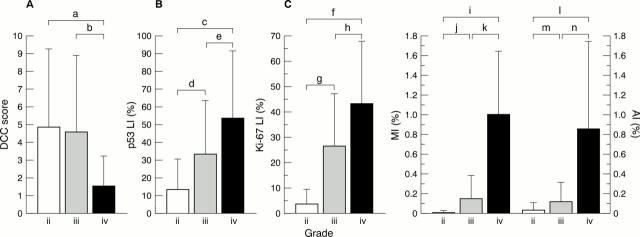
Figure 3 (A) DCC (deleted in colorectal carcinoma) immunoreactivity scores; (B) p53 labelling index (LI); and (C) Ki-67 LI, mitotic index (MI), and apoptotic index (AI). The data are mean (SD) values. a: p = 0.004; b: p = 0.0032; c, f, g, I, k, l, n: p = < 0.0001; d: p = 0.0006; e: p = 0.017; h: p = 0.005; j: p = 0.0013; n: p = 0.0023.
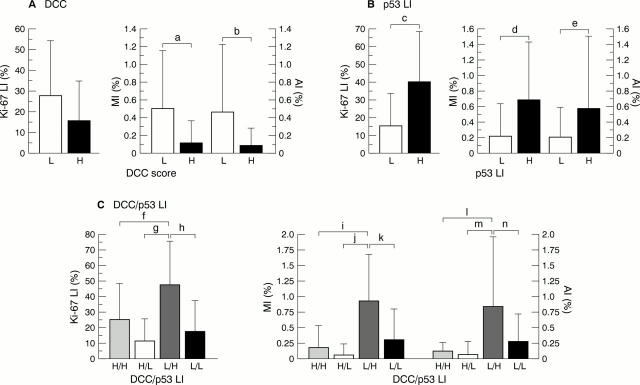
Figure 4 Correlations among immunoreactive scores for (A) DCC (deleted in colorectal carcinoma), (B) p53 labelling index (LI), or (C) DCC/p53 LI combination and Ki-67 LI, mitotic index (MI), or apoptotic index (AI) overall. Subdivisions were into two categories on the basis of average DCC score (mean, 3.7; SD 0.4) and p53 LI (mean, 3.2; SD, 3.1). a: p = 0.0004; b: p = 0.0013; c: p < 0.0001; d: p = 0.0002; e: p = 0.0004; f: p = 0.0168; g: p < 0.0001; h: p < 0.0001; I: p = 0.0004; j: p < 0.0001; k: p < 0.0001; l: p = 0.0024; m: p < 0.0001; n: p = 0.0015. L, low DCC score; H, high DCC score.
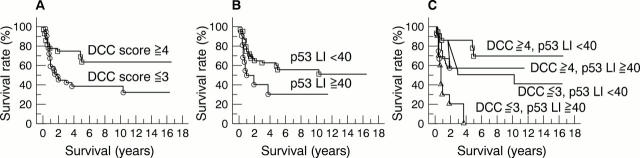
Figure 5 Kaplan-Meier survival curves according to immunoreactivity scores for (A) DCC (deleted in colorectal carcinoma), (B) p53 labelling index (LI), and (C) a combination of the two parameters. (A), p = 0.0371; (B), p = 0.021; (C), p = 0.0017.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardo M. D., Elledge R. M., de Moor C., Clark G. M., Osborne C. K., Allred D. C. bcl-2 and apoptosis in lymph node positive breast carcinoma. Cancer. 1998 Apr 1;82(7):1296–1302. [PubMed] [Google Scholar]
- Cho K. R., Oliner J. D., Simons J. W., Hedrick L., Fearon E. R., Preisinger A. C., Hedge P., Silverman G. A., Vogelstein B. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics. 1994 Feb;19(3):525–531. doi: 10.1006/geno.1994.1102. [DOI] [PubMed] [Google Scholar]
- Chuong C. M., Jiang T. X., Yin E., Widelitz R. B. cDCC (chicken homologue to a gene deleted in colorectal carcinoma) is an epithelial adhesion molecule expressed in the basal cells and involved in epithelial-mesenchymal interaction. Dev Biol. 1994 Aug;164(2):383–397. doi: 10.1006/dbio.1994.1208. [DOI] [PubMed] [Google Scholar]
- Ekstrand B. C., Mansfield T. A., Bigner S. H., Fearon E. R. DCC expression is altered by multiple mechanisms in brain tumours. Oncogene. 1995 Dec 7;11(11):2393–2402. [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Gao X., Honn K. V., Grignon D., Sakr W., Chen Y. Q. Frequent loss of expression and loss of heterozygosity of the putative tumor suppressor gene DCC in prostatic carcinomas. Cancer Res. 1993 Jun 15;53(12):2723–2727. [PubMed] [Google Scholar]
- Hedrick L., Cho K. R., Fearon E. R., Wu T. C., Kinzler K. W., Vogelstein B. The DCC gene product in cellular differentiation and colorectal tumorigenesis. Genes Dev. 1994 May 15;8(10):1174–1183. doi: 10.1101/gad.8.10.1174. [DOI] [PubMed] [Google Scholar]
- Huang Y., Boynton R. F., Blount P. L., Silverstein R. J., Yin J., Tong Y., McDaniel T. K., Newkirk C., Resau J. H., Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992 Dec 1;52(23):6525–6530. [PubMed] [Google Scholar]
- Jaros E., Perry R. H., Adam L., Kelly P. J., Crawford P. J., Kalbag R. M., Mendelow A. D., Sengupta R. P., Pearson A. D. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992 Aug;66(2):373–385. doi: 10.1038/bjc.1992.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996 Oct 18;87(2):175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Soylemezoglu F., Schäuble B., Scheithauer B. W., Burger P. C. Histopathology, classification, and grading of gliomas. Glia. 1995 Nov;15(3):211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- Komaki R., Fujii T., Perkins P., Ro J. Y., Allen P. K., Mason K. A., Mountain C. F., Milas L. Apoptosis and mitosis as prognostic factors in pathologically staged N1 nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 1996 Oct 1;36(3):601–605. doi: 10.1016/s0360-3016(96)00351-3. [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P., Christodoulou P., Kouzelis K., Hadjiyannakis M., Priftis A., Stamoulis G., Seretis A., Thomas-Tsagli E. MDM2 and p53 expression in gliomas: a multivariate survival analysis including proliferation markers and epidermal growth factor receptor. Br J Cancer. 1997;75(9):1269–1278. doi: 10.1038/bjc.1997.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov A., Golanov A., Sycheva R., Pronin I. Prognostic value of tumour associated antigen immunoreactivity and apoptosis in cerebral glioblastomas: an analysis of 168 cases. J Clin Pathol. 1999 Aug;52(8):574–580. doi: 10.1136/jcp.52.8.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis A. P., Bondy M. L., Hess K. R., Cunningham J. E., Zhu D., Amos C. J., Yung W. K., Levin V. A., Bruner J. M. Prognostic significance of p53 immunoreactivity in patients with glioma. Clin Cancer Res. 1995 Dec;1(12):1617–1622. [PubMed] [Google Scholar]
- Louis D. N., Gusella J. F. A tiger behind many doors: multiple genetic pathways to malignant glioma. Trends Genet. 1995 Oct;11(10):412–415. doi: 10.1016/s0168-9525(00)89125-8. [DOI] [PubMed] [Google Scholar]
- Miyake K., Inokuchi K., Dan K., Nomura T. Alterations in the deleted in colorectal carcinoma gene in human primary leukemia. Blood. 1993 Aug 1;82(3):927–930. [PubMed] [Google Scholar]
- Nakatani K., Yoshimi N., Mori H., Sakai H., Shinoda J., Andoh T., Sakai N. The significance of the expression of tumor suppressor gene DCC in human gliomas. J Neurooncol. 1998 Dec;40(3):237–242. doi: 10.1023/a:1006114328134. [DOI] [PubMed] [Google Scholar]
- Reale M. A., Hu G., Zafar A. I., Getzenberg R. H., Levine S. M., Fearon E. R. Expression and alternative splicing of the deleted in colorectal cancer (DCC) gene in normal and malignant tissues. Cancer Res. 1994 Aug 15;54(16):4493–4501. [PubMed] [Google Scholar]
- Reyes-Mugica M., Rieger-Christ K., Ohgaki H., Ekstrand B. C., Helie M., Kleinman G., Yahanda A., Fearon E. R., Kleihues P., Reale M. A. Loss of DCC expression and glioma progression. Cancer Res. 1997 Feb 1;57(3):382–386. [PubMed] [Google Scholar]
- Saegusa M., Hashimura M., Hara A., Okayasu I. Loss of expression of the gene deleted in colon carcinoma (DCC) is closely related to histologic differentiation and lymph node metastasis in endometrial carcinoma. Cancer. 1999 Jan 15;85(2):453–464. doi: 10.1002/(sici)1097-0142(19990115)85:2<453::aid-cncr25>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Saegusa M., Machida D., Okayasu I. Loss of DCC gene expression during ovarian tumorigenesis: relation to tumour differentiation and progression. Br J Cancer. 2000 Feb;82(3):571–578. doi: 10.1054/bjoc.1999.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen P. K., Haapasalo H. K., Visakorpi T., Helén P. T., Rantala I. S., Isola J. J., Helin H. J. Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol. 1994 Dec;174(4):275–282. doi: 10.1002/path.1711740407. [DOI] [PubMed] [Google Scholar]
- Scheck A. C., Coons S. W. Expression of the tumor suppressor gene DCC in human gliomas. Cancer Res. 1993 Dec 1;53(23):5605–5609. [PubMed] [Google Scholar]
- Sinicrope F. A., Ruan S. B., Cleary K. R., Stephens L. C., Lee J. J., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995 Jan 15;55(2):237–241. [PubMed] [Google Scholar]
- Thompson A. M., Morris R. G., Wallace M., Wyllie A. H., Steel C. M., Carter D. C. Allele loss from 5q21 (APC/MCC) and 18q21 (DCC) and DCC mRNA expression in breast cancer. Br J Cancer. 1993 Jul;68(1):64–68. doi: 10.1038/bjc.1993.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S., Tsuda H., Noguchi M., Yokota J., Terada M., Saito T., Kobayashi M., Sugimura T., Hirohashi S. Frequent loss of heterozygosity at the DCC locus in gastric cancer. Cancer Res. 1992 Jun 1;52(11):3099–3102. [PubMed] [Google Scholar]
- Wakimoto H., Aoyagi M., Nakayama T., Nagashima G., Yamamoto S., Tamaki M., Hirakawa K. Prognostic significance of Ki-67 labeling indices obtained using MIB-1 monoclonal antibody in patients with supratentorial astrocytomas. Cancer. 1996 Jan 15;77(2):373–380. doi: 10.1002/(SICI)1097-0142(19960115)77:2<373::AID-CNCR21>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Yamasaki F., Tokunaga O., Sugimori H. Apoptotic index in ovarian carcinoma: correlation with clinicopathologic factors and prognosis. Gynecol Oncol. 1997 Sep;66(3):439–448. doi: 10.1006/gyno.1997.4783. [DOI] [PubMed] [Google Scholar]