Abstract
Background—Coeliac sprue is a chronic disease, in which there is a characteristic mucosal lesion of the small intestine and impaired nutrient absorption, which improves upon the withdrawal of wheat gliadins and related grain proteins from the diet. Biopsy specimens demonstrate diffuse enteritis with pronounced atrophy or total loss of villi. There is also a long term risk of malignant disease.
Aims—To compare the immunoexpression of DCC (deleted in colon cancer), p53, E-cadherin, and ß-catenin in the duodenal mucosa of children with coeliac disease with that seen in children with no evidence of small intestinal disease.
Methods—To gain more insight into the genetic and immunohistochemical alterations of the duodenal epithelium in coeliac disease, 21 endoscopic biopsies from children with coeliac disease and 10 duodenal biopsies from children without coeliac disease were immunohistochemically evaluated for p53, DCC, E-cadherin, and ß-catenin.
Results—DCC expression was not reduced in patients with coeliac disease compared with those without coeliac disease. p53 positive nuclear immunostaining was seen in seven of the 21 patients with coeliac disease. Positive nuclear staining was seen mainly in the deep and the lateral aspects of the crypts. All patients in the control group were negative for p53. In nine and three of the 21 patients with coeliac disease, respectively, the immunohistochemical expression of E-cadherin and ß-catenin was reduced. However, both E-cadherin and ß-catenin immunostaining in the control group was not altered.
Conclusions—E-cadherin and ß-catenin were reduced in the duodenal epithelium of children with coeliac disease when compared with normal mucosa. p53 was overexpressed in the duodenal mucosa of patients with coeliac disease. The reduced expression of E-cadherin and ß-catenin and p53 overexpression may contribute to the morphological changes seen in the small intestinal mucosa in coeliac disease.
Key Words: coeliac disease • p53 • deleted in colon carcinoma gene • E-cadherin • ß-catenin
Full Text
The Full Text of this article is available as a PDF (218.7 KB).
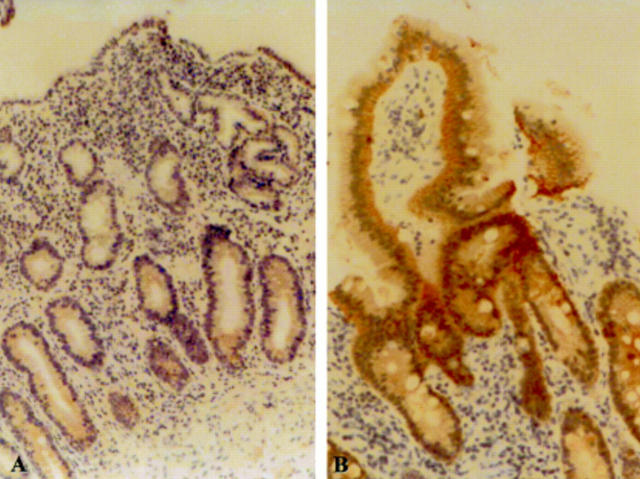
Figure 1 p53 overexpression in the mucosa of a child with coeliac disease at (A) low and (B) high magnification compared with (C) negative p53 staining in normal mucosa.
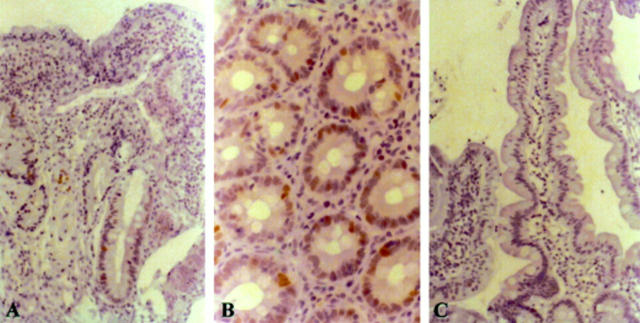
Figure 2 (A) Reduced E-cadherin immunoexpression in the mucosa of coeliac disease (mainly in the surface epithelium) compared with (B) E-cadherin staining in normal mucosa.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André F., Rigot V., Thimonier J., Montixi C., Parat F., Pommier G., Marvaldi J., Luis J. Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int J Cancer. 1999 Nov 12;83(4):497–505. doi: 10.1002/(sici)1097-0215(19991112)83:4<497::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Arber N., Hibshoosh H., Yasui W., Neugut A. I., Hibshoosh A., Yao Y., Sgambato A., Yamamoto H., Shapira I., Rosenman D. Abnormalities in the expression of cell cycle-related proteins in tumors of the small bowel. Cancer Epidemiol Biomarkers Prev. 1999 Dec;8(12):1101–1105. [PubMed] [Google Scholar]
- Barletta A., Marzullo F., Pellecchia A., Montemurro S., Labriola A., Lomonaco R., Grammatica L., Paradiso A. DNA flow cytometry, p53 levels and proliferative cell nuclear antigen in human colon dysplastic, precancerous and cancerous tissues. Anticancer Res. 1998 May-Jun;18(3A):1677–1682. [PubMed] [Google Scholar]
- Bruno C. J., Batts K. P., Ahlquist D. A. Evidence against flat dysplasia as a regional field defect in small bowel adenocarcinoma associated with celiac sprue. Mayo Clin Proc. 1997 Apr;72(4):320–322. doi: 10.4065/72.4.320. [DOI] [PubMed] [Google Scholar]
- Cooper B. T., Holmes G. K., Ferguson R., Cooke W. T. Celiac disease and malignancy. Medicine (Baltimore) 1980 Jul;59(4):249–261. doi: 10.1097/00005792-198007000-00002. [DOI] [PubMed] [Google Scholar]
- Dannenberg A., Godwin T., Rayburn J., Goldin H., Leonard M. Multifocal adenocarcinoma of the proximal small intestine in a patient with celiac sprue. J Clin Gastroenterol. 1989 Feb;11(1):73–76. doi: 10.1097/00004836-198902000-00019. [DOI] [PubMed] [Google Scholar]
- Demetter P., De Vos M., Van Damme N., Baeten D., Elewaut D., Vermeulen S., Mareel M., Bullock G., Mielants H., Verbruggen G. Focal up-regulation of E-cadherin-catenin complex in inflamed bowel mucosa but reduced expression in ulcer-associated cell lineage. Am J Clin Pathol. 2000 Sep;114(3):364–370. doi: 10.1093/ajcp/114.3.364. [DOI] [PubMed] [Google Scholar]
- Doğan A., Wang Z. D., Spencer J. E-cadherin expression in intestinal epithelium. J Clin Pathol. 1995 Feb;48(2):143–146. doi: 10.1136/jcp.48.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. J., Jeejeebhoy K. N., Gopinath N., Girotti M. J., Yeung E. Y., Cullen J. B. Small intestinal villous adenoma and celiac disease. Am J Gastroenterol. 1990 Jun;85(6):748–751. [PubMed] [Google Scholar]
- Fogt F., Vortmeyer A. O., Goldman H., Giordano T. J., Merino M. J., Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998 Feb;29(2):131–136. doi: 10.1016/s0046-8177(98)90222-2. [DOI] [PubMed] [Google Scholar]
- Goi T., Yamaguchi A., Nakagawara G., Urano T., Shiku H., Furukawa K. Reduced expression of deleted colorectal carcinoma (DCC) protein in established colon cancers. Br J Cancer. 1998;77(3):466–471. doi: 10.1038/bjc.1998.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald G. B. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996 Feb 9;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Chinery R., Poulsom R., Playford R. J., Pignatelli M. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. Am J Pathol. 1996 Mar;148(3):723–729. [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Chinery R., Poulsom R., Playford R. J., Pignatelli M. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. Am J Pathol. 1996 Mar;148(3):723–729. [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Holmes G. K., Prior P., Lane M. R., Pope D., Allan R. N. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989 Mar;30(3):333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsken J., Birchmeier W., Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994 Dec;127(6 Pt 2):2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J. A., Bedford F. K., Kim Y. S. Changes in gene structure and regulation of E-cadherin during epithelial development, differentiation, and disease. Prog Nucleic Acid Res Mol Biol. 1997;57:187–215. doi: 10.1016/s0079-6603(08)60281-0. [DOI] [PubMed] [Google Scholar]
- Karayiannakis A. J., Syrigos K. N., Efstathiou J., Valizadeh A., Noda M., Playford R. J., Kmiot W., Pignatelli M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J Pathol. 1998 Aug;185(4):413–418. doi: 10.1002/(SICI)1096-9896(199808)185:4<413::AID-PATH125>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Jensen P. J., Wheelock M. J. Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J Invest Dermatol. 1994 Jun;102(6):870–877. doi: 10.1111/1523-1747.ep12382690. [DOI] [PubMed] [Google Scholar]
- McIntyre A. S., Ng D. P., Smith J. A., Amoah J., Long R. G. The endoscopic appearance of duodenal folds is predictive of untreated adult celiac disease. Gastrointest Endosc. 1992 Mar-Apr;38(2):148–151. doi: 10.1016/s0016-5107(92)70380-0. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Attia L., Scholes J. V., Walters J. R., Holt P. R. Increased small intestinal apoptosis in coeliac disease. Gut. 1996 Dec;39(6):811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohue M., Tomita N., Monden T., Fujita M., Fukunaga M., Takami K., Yana I., Ohnishi T., Enomoto T., Inoue M. A frequent alteration of p53 gene in carcinoma in adenoma of colon. Cancer Res. 1994 Sep 1;54(17):4798–4804. [PubMed] [Google Scholar]
- Perry I., Tselepis C., Hoyland J., Iqbal T. H., Scott D., Sanders S. A., Cooper B. T., Jankowski J. A. Reduced cadherin/catenin complex expression in celiac disease can be reproduced in vitro by cytokine stimulation. Lab Invest. 1999 Dec;79(12):1489–1499. [PubMed] [Google Scholar]
- Pricolo V. E., Mangi A. A., Aswad B., Bland K. I. Gastrointestinal malignancies in patients with celiac sprue. Am J Surg. 1998 Oct;176(4):344–347. doi: 10.1016/s0002-9610(98)00193-7. [DOI] [PubMed] [Google Scholar]
- Przemioslo R., Wright N. A., Elia G., Ciclitira P. J. Analysis of crypt cell proliferation in coeliac disease using MI-B1 antibody shows an increase in growth fraction. Gut. 1995 Jan;36(1):22–27. doi: 10.1136/gut.36.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A., Hamilton S. R. Genetic alterations in sporadic and Crohn's-associated adenocarcinomas of the small intestine. Gastroenterology. 1997 Jul;113(1):127–135. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- Savidge T. C., Shmakov A. N., Walker-Smith J. A., Phillips A. D. Epithelial cell proliferation in childhood enteropathies. Gut. 1996 Aug;39(2):185–193. doi: 10.1136/gut.39.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Reale M. A., Lavin P., Silverman M., Fearon E. R., Steele G., Jr, Jessup J. M., Loda M., Summerhayes I. C. The DCC protein and prognosis in colorectal cancer. N Engl J Med. 1996 Dec 5;335(23):1727–1732. doi: 10.1056/NEJM199612053352303. [DOI] [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Straker R. J., Gunasekaran S., Brady P. G. Adenocarcinoma of the jejunum in association with celiac sprue. J Clin Gastroenterol. 1989 Jun;11(3):320–323. doi: 10.1097/00004836-198906000-00015. [DOI] [PubMed] [Google Scholar]
- Swinson C. M., Slavin G., Coles E. C., Booth C. C. Coeliac disease and malignancy. Lancet. 1983 Jan 15;1(8316):111–115. doi: 10.1016/s0140-6736(83)91754-3. [DOI] [PubMed] [Google Scholar]
- Takayama T., Shiozaki H., Shibamoto S., Oka H., Kimura Y., Tamura S., Inoue M., Monden T., Ito F., Monden M. Beta-catenin expression in human cancers. Am J Pathol. 1996 Jan;148(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- Trier J. S. Celiac sprue. N Engl J Med. 1991 Dec 12;325(24):1709–1719. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- Trier J. S. Diagnosis of celiac sprue. Gastroenterology. 1998 Jul;115(1):211–216. doi: 10.1016/s0016-5085(98)70383-x. [DOI] [PubMed] [Google Scholar]
- Variend S., Placzek M., Raafat F., Walker-Smith J. A. Small intestinal mucosal fat in childhood enteropathies. J Clin Pathol. 1984 Apr;37(4):373–377. doi: 10.1136/jcp.37.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner D. A., Krzeminski K. A., Nelson W. J. Remodeling the cell surface distribution of membrane proteins during the development of epithelial cell polarity. J Cell Biol. 1992 Feb;116(4):889–899. doi: 10.1083/jcb.116.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wurff A. A., ten Kate J., van der Linden E. P., Dinjens W. N., Arends J. W., Bosman F. T. L-CAM expression in normal, premalignant, and malignant colon mucosa. J Pathol. 1992 Nov;168(3):287–291. doi: 10.1002/path.1711680308. [DOI] [PubMed] [Google Scholar]