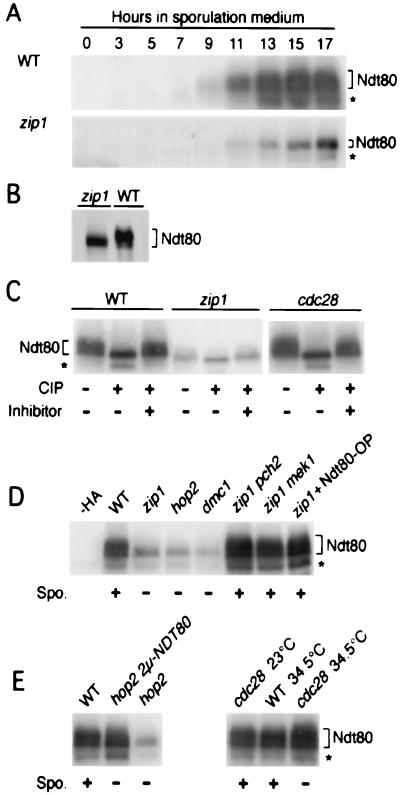
Figure 3.
Phosphorylation of Ndt80 is prevented by the pachytene checkpoint. (A) Production of Ndt80 in wild-type (WT) and zip1∷LEU2 cells was monitored throughout meiosis by Western blot analysis with anti-HA antibodies. (B) Western blot analysis in which the amount of zip1 extract loaded was five times that of wild type; extracts were prepared after 15.5 h of sporulation. (C) Ndt80-HA from wild-type, zip1∷LEU2, and cdc28–63 cells was immunoprecipitated, treated with CIP, and analyzed by immunoblotting. cdc28–63 cells were grown at 23°C, incubated in sporulation medium at 23° for 3 h, and then shifted to 34.5°C. (D) Immunoblot analysis of Ndt80-HA in wild type, zip1∷LEU2, hop2∷URA3, dmc1∷LEU2, zip1∷LEU2 pch2∷URA3, zip1∷LEU2 mek1∷URA3, and zip1∷LEU2 containing a multicopy plasmid carrying NDT80-HA (pTP118). (E) Immunoblot analysis of Ndt80-HA in wild type, hop2∷LEU2 carrying the NDT80-HA multicopy plasmid (pTP118), hop2∷URA3, and cdc28–63. For cdc28 and the wild-type control, cells were grown at 23°C, incubated in sporulation medium for 3 h, and then either shifted to 34.5°C or maintained at 23°C, as indicated. The + and − signs below each lane in D and E indicate the ability of the strain to sporulate (Spo.). Equal amounts of extract (≈30 μg) were loaded per lane, except in B. Unless otherwise noted, cells were harvested after 17 h in sporulation medium. All strains are isogenic with BR2495 and homozygous for NDT80-HA. The band indicated by an asterisk is presumed to be a degradation product of Ndt80.