Abstract
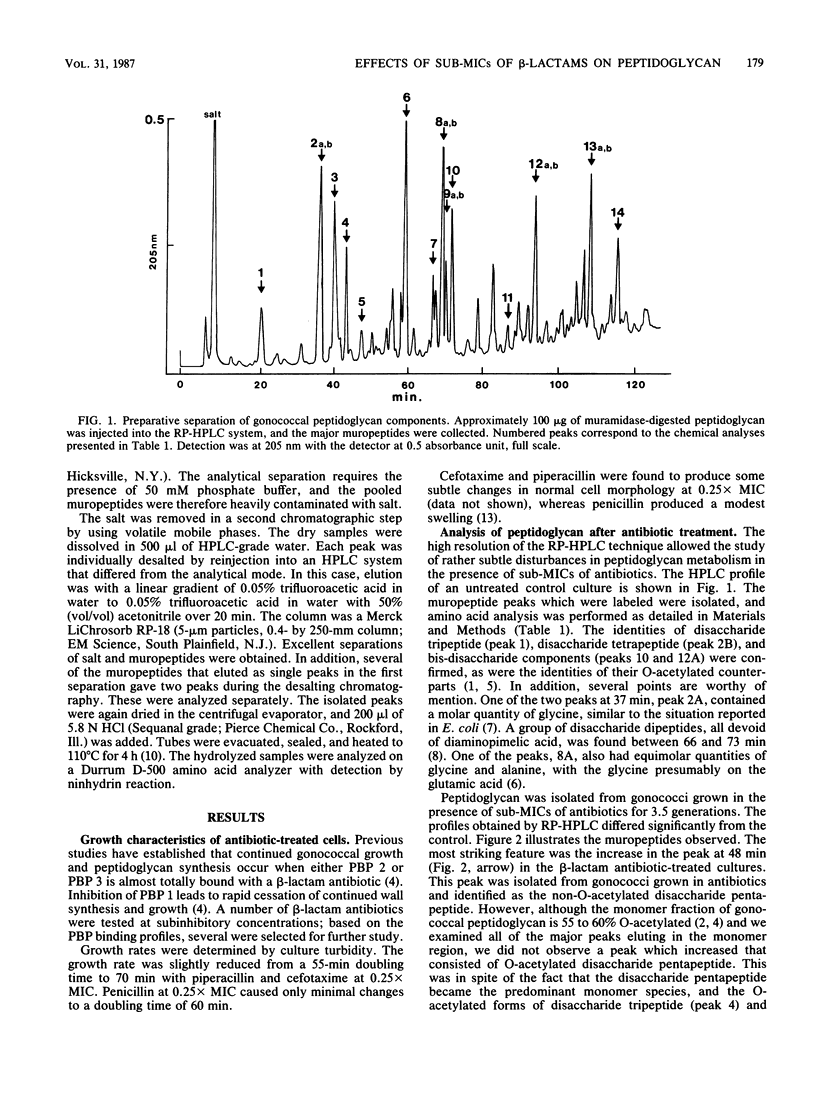
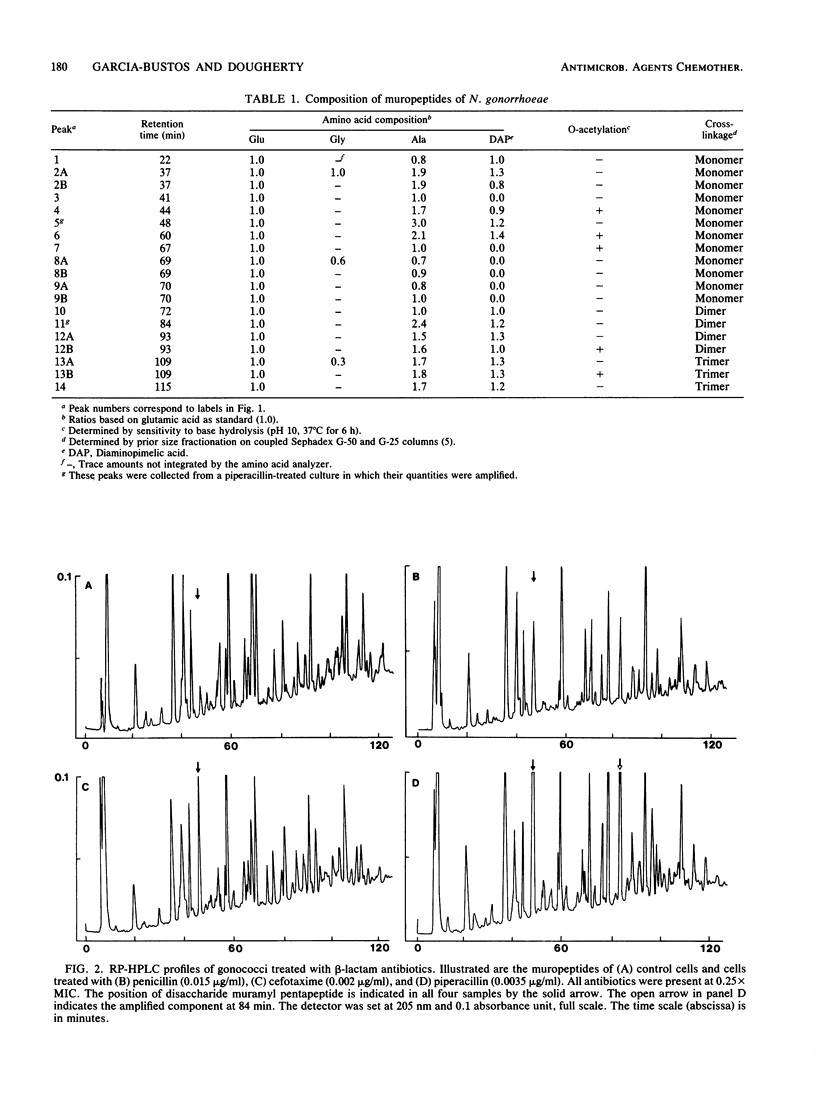
Exposure of Neisseria gonorrhoeae to sub-MICs of selected beta-lactam antibiotics caused distortion of normal cell morphology. Analysis of the peptidoglycan indicated that the cells were accumulating increased quantities of disaccharide pentapeptide in their cell walls. The O-acetylated form of the disaccharide pentapeptide was not detected among the major peaks. The correlation of antibiotic binding to gonococcal penicillin-binding protein 2 and accumulation of non-O-acetylated disaccharide pentapeptide suggested an explanation for the previously observed relationship of penicillin-binding protein 2 and O-acetylation of peptidoglycan.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell J. K., Perkins H. R. The peptidoglycan of Neisseria gonorrhoeae, with or without O-acetyl groups, contains anhydro-muramyl residues. J Gen Microbiol. 1985 Dec;131(12):3397–3400. doi: 10.1099/00221287-131-12-3397. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J Bacteriol. 1985 Jul;163(1):69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Involvement of a change in penicillin target and peptidoglycan structure in low-level resistance to beta-lactam antibiotics in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1985 Jul;28(1):90–95. doi: 10.1128/aac.28.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Essig P., Martin H. H. Characterization of minor fragments after digestion of Escherichia coli murein with endo-N,O-diacetylmuramidase from Chalaropsis, and determination of glycan chain length. FEBS Lett. 1982 Feb 8;138(1):109–112. doi: 10.1016/0014-5793(82)80406-7. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Markiewicz Z., Schwarz U. Absence of oligomeric murein intermediates in Escherichia coli. J Bacteriol. 1983 Oct;156(1):130–135. doi: 10.1128/jb.156.1.130-135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear A. L., Perkins H. R. Degrees of O-acetylation and cross-linking of the peptidoglycan of Neisseria gonorrhoeae during growth. J Gen Microbiol. 1983 Mar;129(3):885–888. doi: 10.1099/00221287-129-3-885. [DOI] [PubMed] [Google Scholar]
- Lorian V., Atkinson B. Effects of subinhibitory concentrations of antibiotics on cross walls of cocci. Antimicrob Agents Chemother. 1976 Jun;9(6):1043–1055. doi: 10.1128/aac.9.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett H., Bracha R., Mirelman D. Soluble nascent peptidoglycan in growing Escherichia coli cells. J Biol Chem. 1980 Oct 25;255(20):9884–9890. [PubMed] [Google Scholar]
- Rosenthal R. S., Gfell M. A., Folkening W. J. Influence of protein synthesis inhibitors on regulation of extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1985 Jul;49(1):7–13. doi: 10.1128/iai.49.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):273–283. doi: 10.1098/rstb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


