Abstract
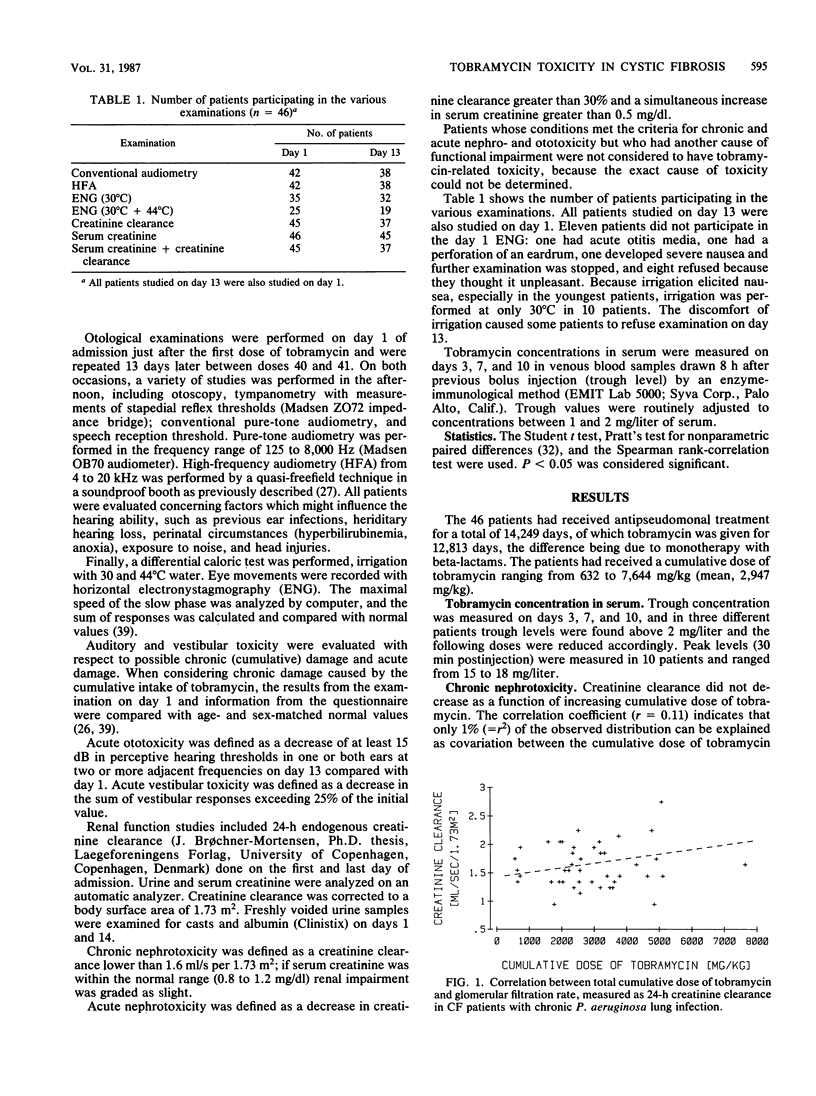
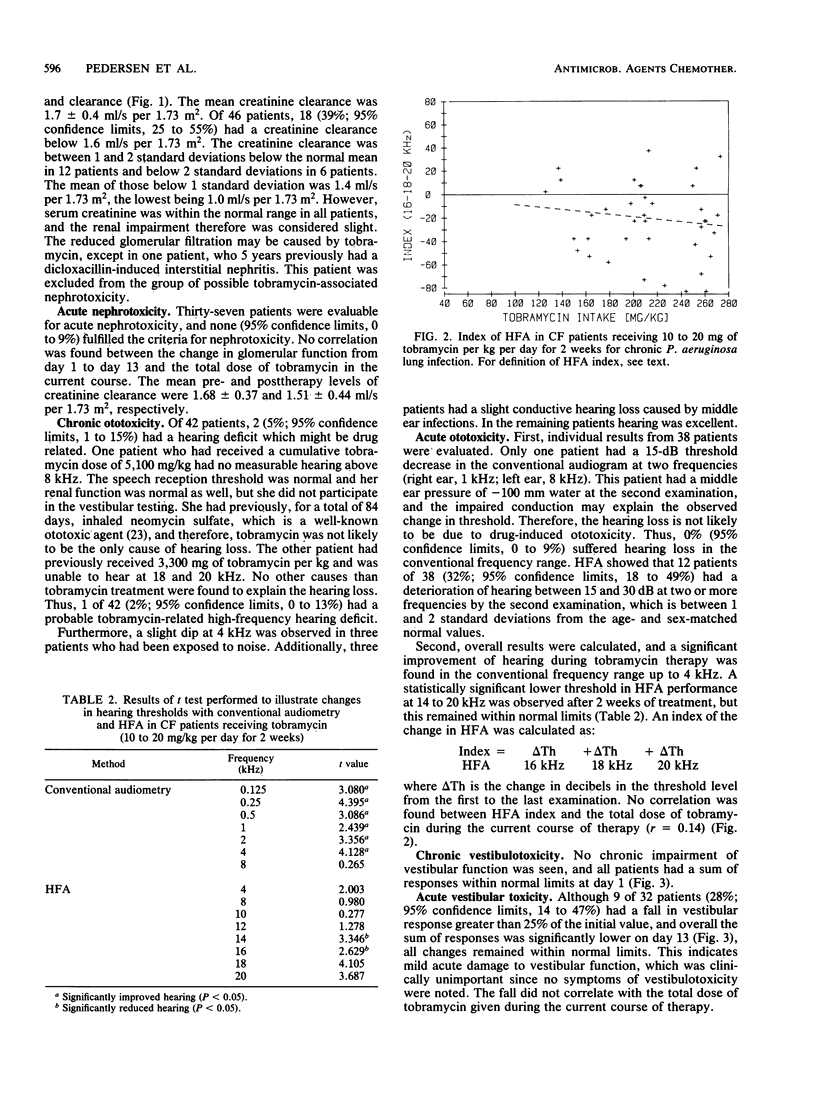
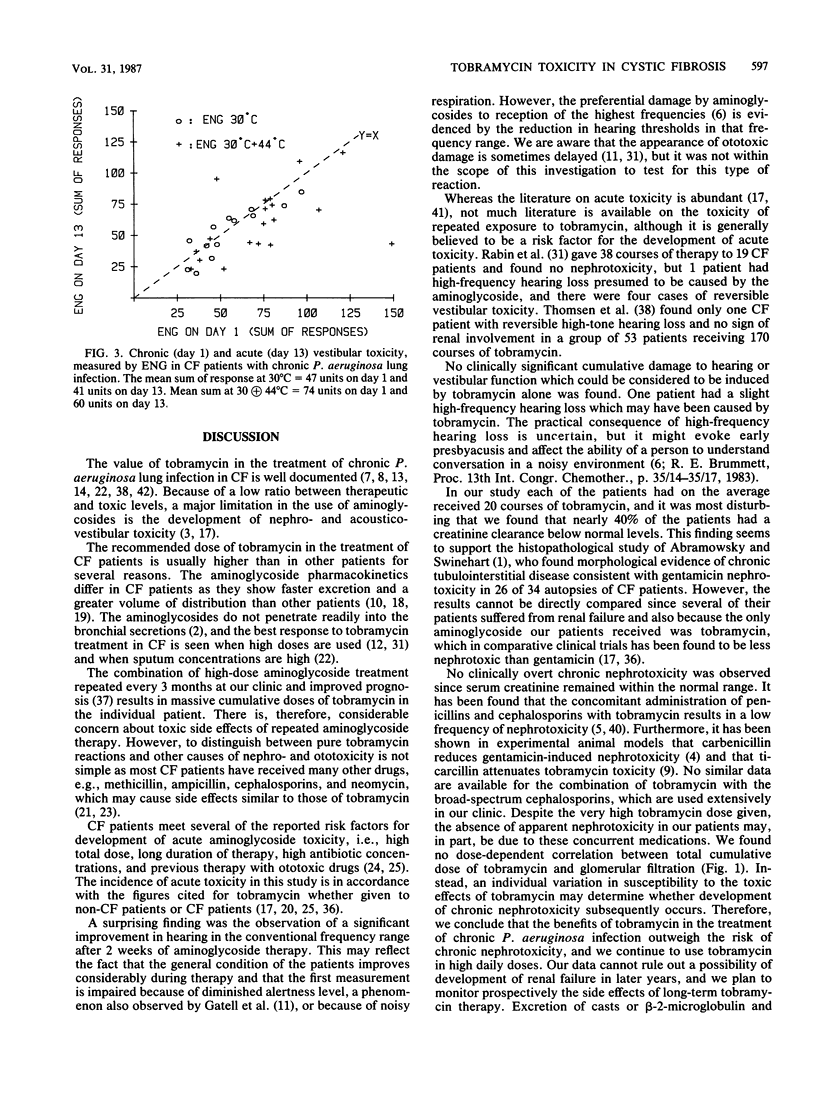
Forty-six patients with cystic fibrosis and chronic bronchopulmonary Pseudomonas aeruginosa infection entered a study of tobramycin-related chronic and acute nephro- and acousticovestibular toxicity. The patients (mean age, 15.7 years) had previously received 2-week courses of tobramycin therapy, for a mean cumulative total of 279 days each. The cumulative tobramycin dose ranged from 632 to 7,644 mg/kg. The patients were studied before and at the end of a 2-week course of treatment with tobramycin (10 to 20 mg/kg per day) to discriminate between acute and chronic toxicity. In patients studied at the beginning of the present course of treatment, the glomerular filtration rate, measured as 24-h creatinine clearance, did not correlate with the cumulative dose of tobramycin received during previous courses. Eighteen patients (39%) had a reduced glomerular filtration rate compared with normal values (mean, 12.5% reduction) but normal serum creatinine values. Two patients (5%) had a high-frequency hearing deficit (above 8 kHz), but only one deficit was possibly related to tobramycin. No chronic vestibular toxicity was observed. During the course of treatment, no patients developed acute nephrotoxicity. After 2 weeks of treatment 32% had a slightly reduced hearing threshold (15 to 30 dB) in two or more high frequencies, and 28% had a fall in vestibular response greater than 25% of the initial value but remained within normal limits. Thus, the acute and chronic toxicity of repeated high-dose tobramycin treatment in cystic fibrosis patients seems to be very mild.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowsky C. R., Swinehart G. L. The nephropathy of cystic fibrosis: a human model of chronic nephrotoxicity. Hum Pathol. 1982 Oct;13(10):934–939. doi: 10.1016/s0046-8177(82)80056-7. [DOI] [PubMed] [Google Scholar]
- Bloch R., Luft F. C., Rankin L. I., Sloan R. S., Yum M. N., Maxwell D. R. Protection from gentamicin nephrotoxicity by cephalothin and carbenicillin. Antimicrob Agents Chemother. 1979 Jan;15(1):46–49. doi: 10.1128/aac.15.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Quesada O., Armstrong D. Minimal nephrotoxicity with cephalosporin-aminoglycoside combinations in patients with neoplastic disease. Antimicrob Agents Chemother. 1982 Apr;21(4):592–594. doi: 10.1128/aac.21.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett R. E. Drug-induced ototoxicity. Drugs. 1980 Jun;19(6):412–428. doi: 10.2165/00003495-198019060-00002. [DOI] [PubMed] [Google Scholar]
- Conway S. P., Miller M. G., Ramsden C., Littlewood J. M. Intensive treatment of pseudomonas chest infection in cystic fibrosis: a comparison of tobramycin and ticarcillin, and netilmicin and ticarcillin. Acta Paediatr Scand. 1985 Jan;74(1):107–113. doi: 10.1111/j.1651-2227.1985.tb10929.x. [DOI] [PubMed] [Google Scholar]
- English J., Gilbert D. N., Kohlhepp S., Kohnen P. W., Mayor G., Houghton D. C., Bennett W. M. Attenuation of experimental tobramycin nephrotoxicity by ticarcillin. Antimicrob Agents Chemother. 1985 Jun;27(6):897–902. doi: 10.1128/aac.27.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Hall K. Aminoglycoside clearance in patients with cystic fibrosis. J Pediatr. 1979 Jan;94(1):163–164. doi: 10.1016/s0022-3476(79)80394-7. [DOI] [PubMed] [Google Scholar]
- Gatell J. M., SanMiguel J. G., Araujo V., Zamora L., Maña J., Ferrer M., Bonet M., Bohe M., Jimenez de Anta M. T. Prospective randomized double-blind comparison of nephrotoxicity and auditory toxicity of tobramycin and netilmicin. Antimicrob Agents Chemother. 1984 Nov;26(5):766–769. doi: 10.1128/aac.26.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley H. B., Lewis R. M., Swartz D. R., Gump D. W. Tobramycin therapy of pulmonary infections in patients with cystic fibrosis. Curr Ther Res Clin Exp. 1974 May;16(5):414–423. [PubMed] [Google Scholar]
- Hoff G. E., Schiotz P. O., Paulsen J. Tobramycin treatment of pseudomonas aeruginosa infections in cystic fibrosis. Scand J Infect Dis. 1974;6(4):333–337. doi: 10.3109/inf.1974.6.issue-4.07. [DOI] [PubMed] [Google Scholar]
- Kahlmeter G., Dahlager J. I. Aminoglycoside toxicity - a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984 Jan;13 (Suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Kearns G. L., Hilman B. C., Wilson J. T. Dosing implications of altered gentamicin disposition in patients with cystic fibrosis. J Pediatr. 1982 Feb;100(2):312–318. doi: 10.1016/s0022-3476(82)80663-x. [DOI] [PubMed] [Google Scholar]
- Kelly H. B., Menendez R., Fan L., Murphy S. Pharmacokinetics of tobramycin in cystic fibrosis. J Pediatr. 1982 Feb;100(2):318–321. doi: 10.1016/s0022-3476(82)80664-1. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Reyes M. P., Cone L. A., Blair D. C., Jansen W., Wright G. E., Lorber R. R. Randomised, controlled trial of the comparative efficacy, auditory toxicity, and nephrotoxicity of tobramycin and netilmicin. Lancet. 1983 May 21;1(8334):1123–1126. doi: 10.1016/s0140-6736(83)92864-7. [DOI] [PubMed] [Google Scholar]
- Linton A. L., Clark W. F., Driedger A. A., Turnbull D. I., Lindsay R. M. Acute interstitial nephritis due to drugs: Review of the literature with a report of nine cases. Ann Intern Med. 1980 Nov;93(5):735–741. doi: 10.7326/0003-4819-93-5-735. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lietman P. S. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis. 1984 Jan;149(1):23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- Osterhammel D., Osterhammel P. High-frequency audiometry. Age and sex variations. Scand Audiol. 1979;8(2):73–81. doi: 10.3109/01050397909076304. [DOI] [PubMed] [Google Scholar]
- Osterhammel D., Osterhammel P., Terkildsen K. A quasi-free-field transducer system for high-frequency audiometry. Scand Audiol. 1977;6(2):91–95. doi: 10.3109/01050397709045004. [DOI] [PubMed] [Google Scholar]
- Pedersen S. S., Koch C., Høiby N., Rosendal K. An epidemic spread of multiresistant Pseudomonas aeruginosa in a cystic fibrosis centre. J Antimicrob Chemother. 1986 Apr;17(4):505–516. doi: 10.1093/jac/17.4.505. [DOI] [PubMed] [Google Scholar]
- Phillips I. Good antimicrobial prescribing. Aminoglycosides. Lancet. 1982 Aug 7;2(8293):311–314. doi: 10.1016/s0140-6736(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Reed M. D., Vermeulen M. W., Stern R. C., Cheng P. W., Powell S. H., Boat T. F. Are measurements of urine enzymes useful during aminoglycoside therapy? Pediatr Res. 1981 Sep;15(9):1234–1239. doi: 10.1203/00006450-198109000-00002. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Cerra F. B., Plaut M. E. Clinical and pharmacokinetic characteristics of aminoglycoside nephrotoxicity in 201 critically ill patients. Antimicrob Agents Chemother. 1982 May;21(5):721–726. doi: 10.1128/aac.21.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. R., Lipsky J. J., Laskin O. L., Hellmann D. B., Mellits E. D., Longstreth J., Lietman P. S. Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 1980 May 15;302(20):1106–1109. doi: 10.1056/NEJM198005153022002. [DOI] [PubMed] [Google Scholar]
- Szaff M., Høiby N., Flensborg E. W. Frequent antibiotic therapy improves survival of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection. Acta Paediatr Scand. 1983 Sep;72(5):651–657. doi: 10.1111/j.1651-2227.1983.tb09789.x. [DOI] [PubMed] [Google Scholar]
- Thomsen J., Friis B., Jensen K., Bak-Pedersen K., Larsen P. K. Tobramycin ototoxicity. Repeated courses of high dosage treatment in children with cystic fibrosis. J Antimicrob Chemother. 1979 May;5(3):257–260. doi: 10.1093/jac/5.3.257. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Wiernik P. H. Antibiotic combination-associated nephrotoxicity in granulocytopenic patients with cancer. Arch Intern Med. 1981 Dec;141(13):1789–1793. doi: 10.1001/archinte.141.13.1789. [DOI] [PubMed] [Google Scholar]
- Wersäll J. Recent otological evaluation of aminoglycoside antibiotics. J Antimicrob Chemother. 1984 Jan;13 (Suppl A):31–36. doi: 10.1093/jac/13.suppl_a.31. [DOI] [PubMed] [Google Scholar]
- Wientzen R., Prestidge C. B., Kramer R. I., McCracken G. H., Nelson J. D. Acute pulmonary exacerbations in cystic fibrosis. A double-blind trial of tobramycin and placebo therapy. Am J Dis Child. 1980 Dec;134(12):1134–1138. doi: 10.1001/archpedi.1980.02130240018007. [DOI] [PubMed] [Google Scholar]


