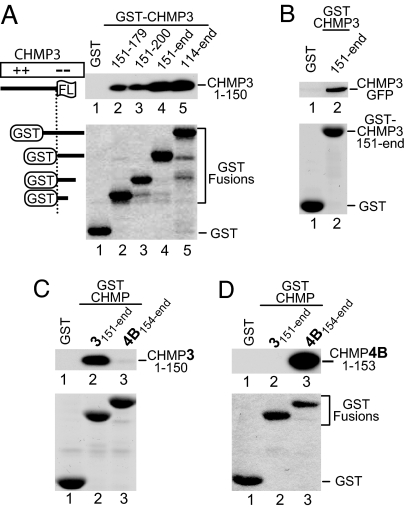
Fig. 2.
Dominant-negative CHMP truncation mutants specifically bind to nonoverlapping fragments from their cognate acidic domains. (A) Binding of dominant-negative CHMP31–150FLAG to CHMP3 acidic domain fragments. Beads decorated with the indicated GST fusion proteins or GST were incubated with lysates from 293T cells expressing CHMP31–150FLAG. The levels of the GST fusion proteins on the beads were monitored by SDS/PAGE and Coomassie staining. Captured CHMP31–150FLAG was detected by Western blotting with anti-FLAG antibody. (B) GST pull-down showing that full-length CHMP3 fused to GFP binds to the acidic domain fragment CHMP3151–end. (C) GST pull-down showing that dominant-negative CHMP31–150FLAG binds to the nonoverlapping CHMP3151–end fragment but not to the corresponding region of CHMP4B. (D) Conversely, dominant-negative CHMP4B1–153FLAG binds to CHMP4B154–end but not to the corresponding region of CHMP3.