Fig. 3.
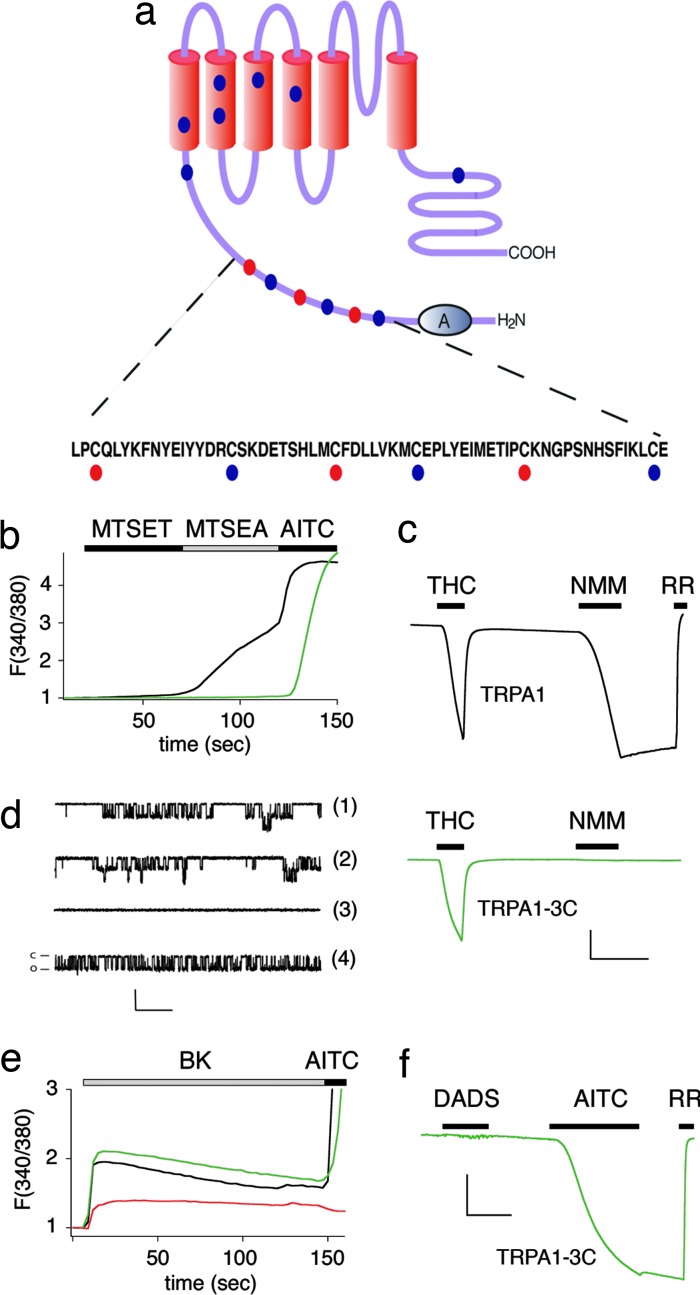
Three cysteine residues in the intracellular N terminus of TRPA1 confer sensitivity to NMM. (a) Cysteine mutations conferring sensitivity to NMM cluster within a 50-aa stretch between the cluster of 18 ankyrin repeats and the first putative transmembrane region. Red dots denote residues required for full response to sulfhydryl modification reagents. Blue dots denote sites where mutations had no effect. (b) Activation of wild-type hTRPA1 (black trace) and triple mutant hTRPA1-3C (green trace) by sulfhydryl-selective reagents show expected properties for an intracellular reactive site. Membrane-impermeable reagent MTSET (200 μM) fails to activate either channel, whereas membrane-permeant MTSEA (200 μM) activates wild-type hTRPA1 only. AITC (200 μM) is used as a positive control. Traces represent normalized average calcium response of transfected HEK293 cells (n = 350) as assessed by Fura-2/AM ratiometric calcium imaging. Mean peak responses ± SEM: MTSET, 1.09 ± 0.01 (WT), 1.02 ± 0.0003 (3C); MTSEA, 2.34 ± 0.07 (WT), 1.03 ± 0.01 (3C); AITC, 4.73 ± 0.05 (WT), 4.87 ± 0.50 (3C). (c) Representative traces (–80 mV) from an oocyte expressing wild-type hTRPA1 (black) compared with NMM-insensitive triple mutant hTRPA1-3C (green). THC (400 μM, with 200 μM β-cyclodextran carrier) elicits robust, reversible inward currents in both cases, whereas NMM (50 μM) does not appreciably activate the triple mutant (n = 5 for each trace). (Scale bars: 200 nA and 1 min.) (d) NMM (50 μM, trace 1) activates human TRPA1 in a similar manner to AITC (20 μM, trace 2). For traces 1 and 2, HEK293 cells stably expressing hTRPA1 were recorded under cell-attached configuration at a holding potential of –60 mV, with sampling frequency of 5 kHz, filtering at 1 kHZ. Single-channel conductances (±SD) were as follows: 102.7 ± 12.9 for NMM (n = 4) and 101.8 ± 10.9 pS (n = 4) for AITC. Agonists were delivered by backfilling pipet with drug. For traces 3 and 4, oocytes injected with TRPA1-3C were recorded under similar conditions. Activation of TRPA1 by NMM (50 μM) is specifically abrogated in triple mutant hTRPA1-3C (trace 3), whereas AITC (500 μM) still can activate this current (trace 4). Single-channel conductance (±SD) was 107.7 ± 10.7 pS (n = 6) with 500 μM AITC backfilled in the pipet. (Scale bars: 10 pA and 200 msec.) (e) Triple mutant TRPA1-3C retains receptor-operated channel function. Average traces of calcium response from transfected HEK 293 cells (n = 50, two trials), as assessed by Fura-2/AM imaging. Upon application of bradykinin (BK) (100 nM), cells cotransfected with BK2R and wild-type hTRPA1 (black trace) or triple mutant hTRPA1-3C (green trace) show sustained elevated calcium levels in excess of cells transfected with BK2R alone (red trace). Mean peak responses: 2.52 ± 0.18 (WT + BK2R), 2.80 ± 0.23 (3C + BK2R), and 1.29 ± 0.20 (BK2R alone). (f) Triple mutant hTRPA1-3C is not responsive to diallyl disulfide (DADS) (600 μM). Representative trace from oocytes expressing triple mutant hTRPA1-3C (n = 5). Response to AITC (200 μM) is used as a positive control. (Scale bars: 100 nA and 1 min.)