Abstract
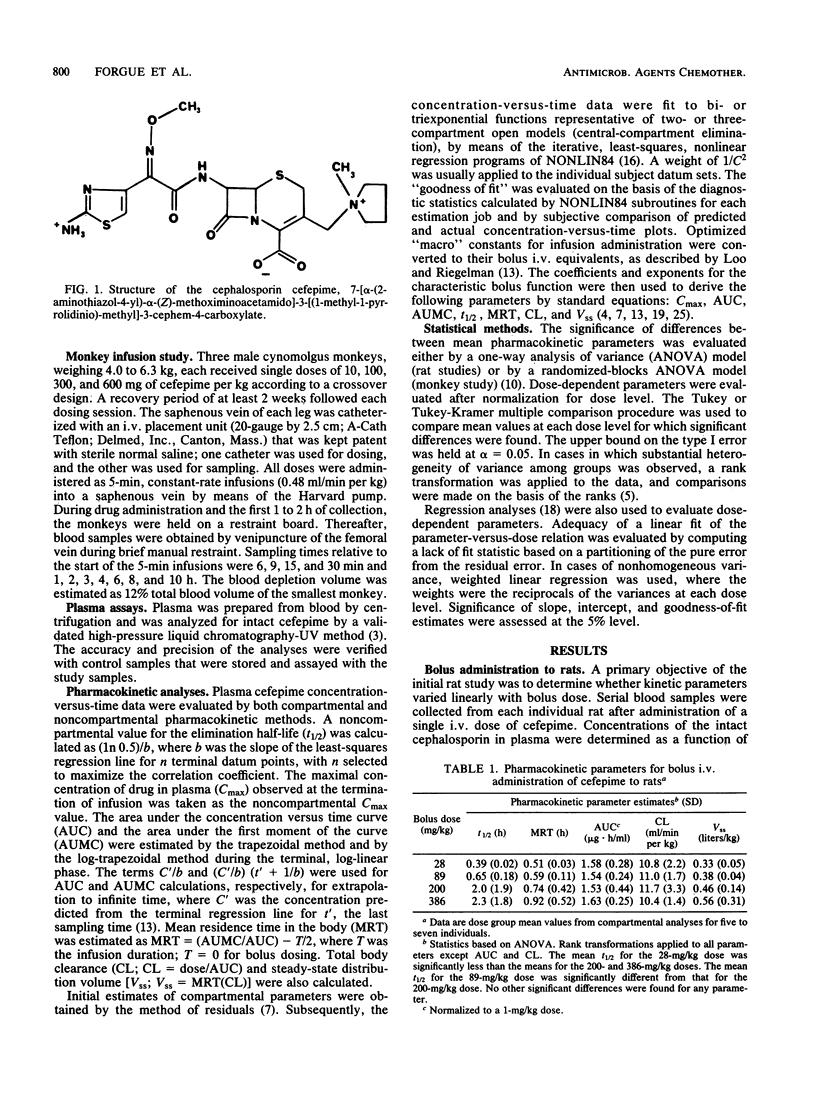
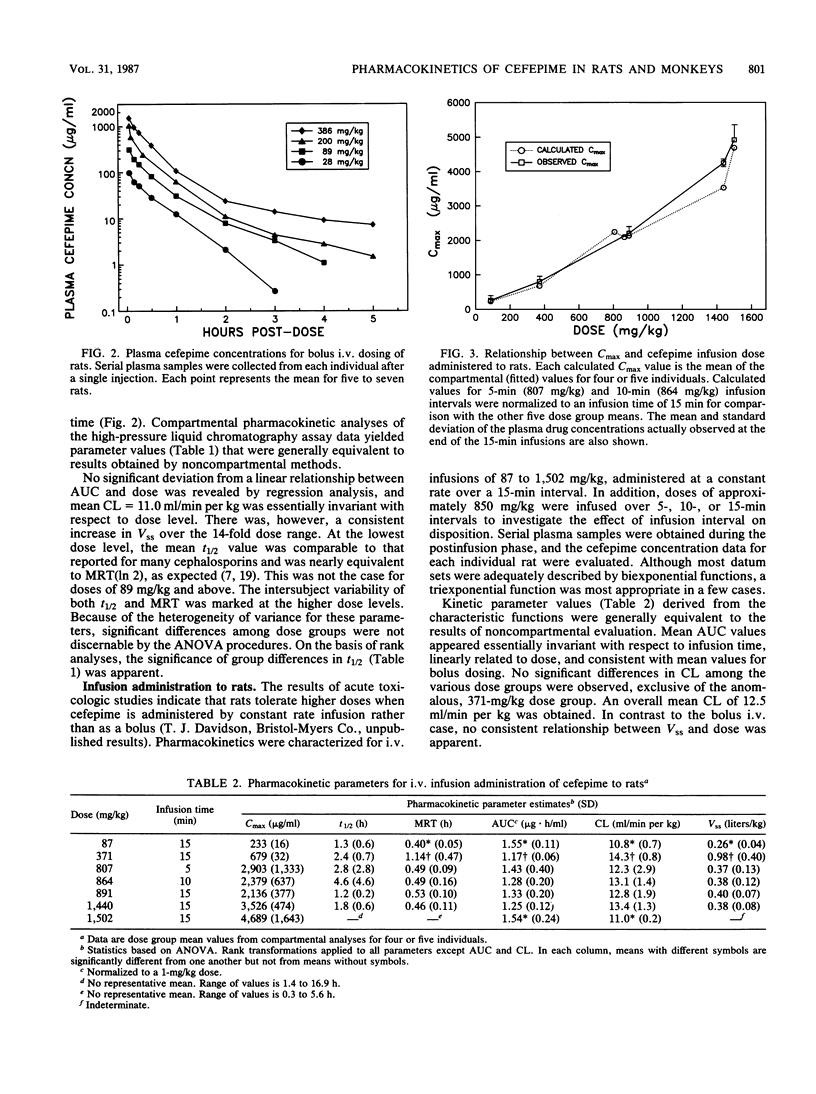
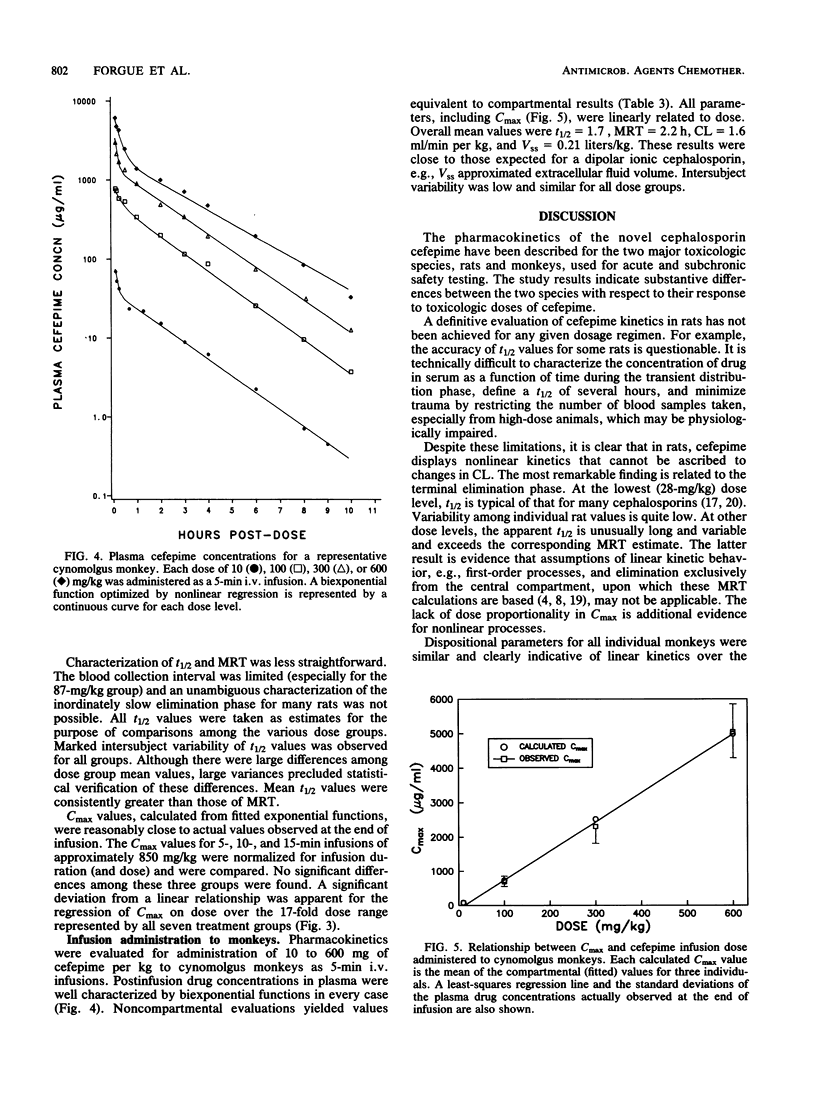
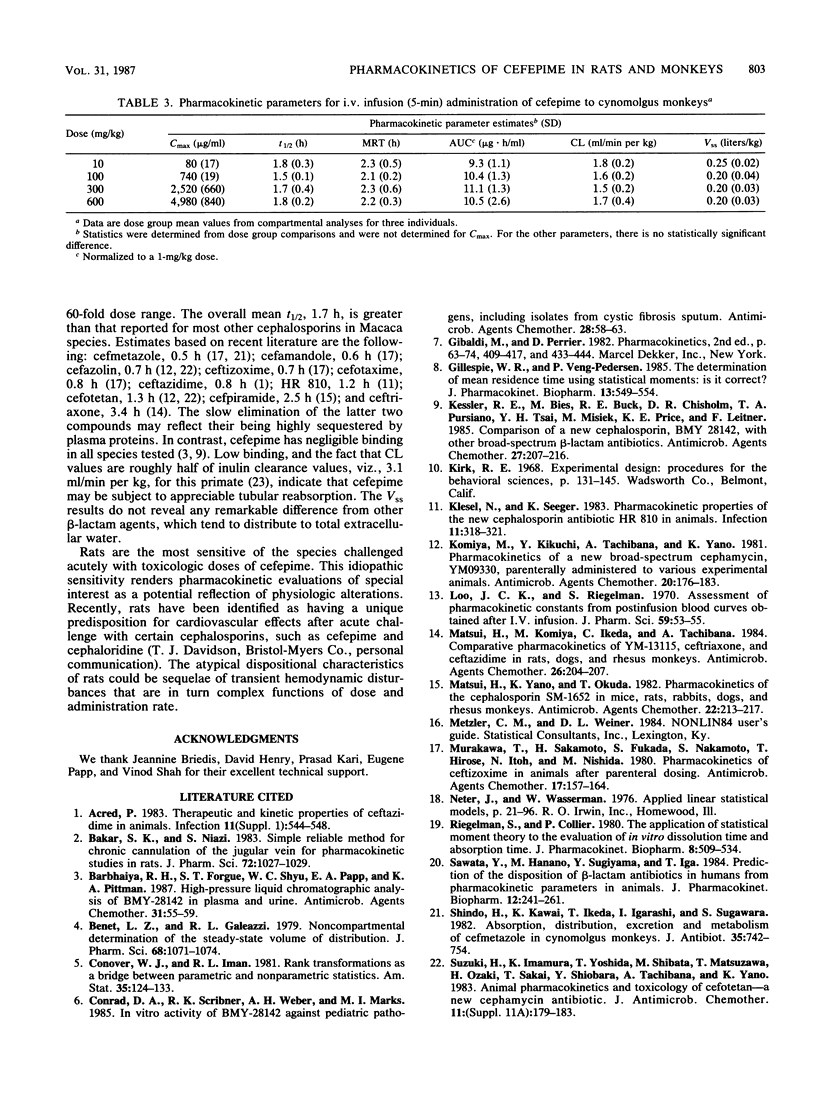
The disposition of the novel cephalosporin cefepime (BMY-28142) was characterized for intravenous administration of single doses to rats and cynomolgus monkeys, the species used most extensively for safety evaluation of the compound. Serial blood samples were collected from individual animals, and plasma was analyzed for intact cefepime by a high-pressure liquid chromatography-UV method. Assay results were evaluated by compartmental and noncompartmental methods to characterize pharmacokinetics for each species and dosage regimen. For intravenous (i.v.) bolus administration of 28 to 386 mg/kg (body weight) to rats, total body clearance (CL; 11.0 ml/min per kg) was essentially invariant with the dose; however, the terminal half-life (t1/2) and the steady-state distribution volume (Vss) increased with increasing dose level. After administration of 87 to 1,502 mg/kg by i.v. infusion, CL (12.5 ml/min per kg) was again similar for all dose groups. Mean t1/2 values (1.3 to 4.6 h) appeared unusually long for a cephalosporin in rats, and inordinately variable. No consistent differences among dose group mean Vss values were found. The maximal concentration of drug in plasma at the end of infusion was not a linear function of dose. For the cynomolgus monkey, kinetic parameters for 5-min i.v. infusions were linearly related to dose over the range of 10 to 600 mg/kg. Mean parameter values were t1/2 = 1.7 h, CL = 1.6 ml/min per kg, and Vss = 0.21 liters/kg. The pharmacokinetic results indicate substantive differences between the two species with respect to their response to toxicologic doses of cefepime.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakar S. K., Niazi S. Simple reliable method for chronic cannulation of the jugular vein for pharmacokinetic studies in rats. J Pharm Sci. 1983 Sep;72(9):1027–1029. doi: 10.1002/jps.2600720915. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Conrad D. A., Scribner R. K., Weber A. H., Marks M. I. In vitro activity of BMY-28142 against pediatric pathogens, including isolates from cystic fibrosis sputum. Antimicrob Agents Chemother. 1985 Jul;28(1):58–63. doi: 10.1128/aac.28.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie W. R., Veng-Pedersen P. The determination of mean residence time using statistical moments: it is correct. J Pharmacokinet Biopharm. 1985 Oct;13(5):549–554. doi: 10.1007/BF01059336. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesel N., Seeger K. Pharmacokinetic properties of the new cephalosporin antibiotic HR 810 in animals. Infection. 1983 Nov-Dec;11(6):318–321. doi: 10.1007/BF01641356. [DOI] [PubMed] [Google Scholar]
- Komiya M., Kikuchi Y., Tachibana A., Yano K. Pharmacokinetics of new broad-spectrum cephamycin, YM09330, parenterally administered to various experimental animals. Antimicrob Agents Chemother. 1981 Aug;20(2):176–183. doi: 10.1128/aac.20.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Matsui H., Komiya M., Ikeda C., Tachibana A. Comparative pharmacokinetics of YM-13115, ceftriaxone, and ceftazidime in rats, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1984 Aug;26(2):204–207. doi: 10.1128/aac.26.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Yano K., Okuda T. Pharmacokinetics of the cephalosporin SM-1652 in mice, rats, rabbits, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1982 Aug;22(2):213–217. doi: 10.1128/aac.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T., Sakamoto H., Fukada S., Nakamoto S., Hirose T., Itoh N., Nishida M. Pharmacokinetics of ceftizoxime in animals after parenteral dosing. Antimicrob Agents Chemother. 1980 Feb;17(2):157–164. doi: 10.1128/aac.17.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Hanano M., Sugiyama Y., Iga T. Prediction of the disposition of beta-lactam antibiotics in humans from pharmacokinetic parameters in animals. J Pharmacokinet Biopharm. 1984 Jun;12(3):241–261. doi: 10.1007/BF01061720. [DOI] [PubMed] [Google Scholar]
- Shindo H., Kawai K., Ikeda T., Igarashi I., Sugawara S. Absorption, distribution, excretion and metabolism of cefmetazole in cynomolgus monkeys. J Antibiot (Tokyo) 1982 Jun;35(6):742–754. doi: 10.7164/antibiotics.35.742. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Imamura K., Yoshida T., Shibata M., Matsuzawa T., Ozaki H., Sakai T., Shiobara Y., Tachibana A., Yano K. Animal pharmacokinetics and toxicology of cefotetan--a new cephamycin antibiotic. J Antimicrob Chemother. 1983 Jan;11 (Suppl):179–183. doi: 10.1093/jac/11.suppl_a.179. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Maniatis A., Bertram M. A., Young L. S. In vitro activity of BMY-28142 in comparison with those of other beta-lactam antimicrobial agents. Antimicrob Agents Chemother. 1985 Apr;27(4):515–519. doi: 10.1128/aac.27.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokinet Biopharm. 1976 Oct;4(5):443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]


