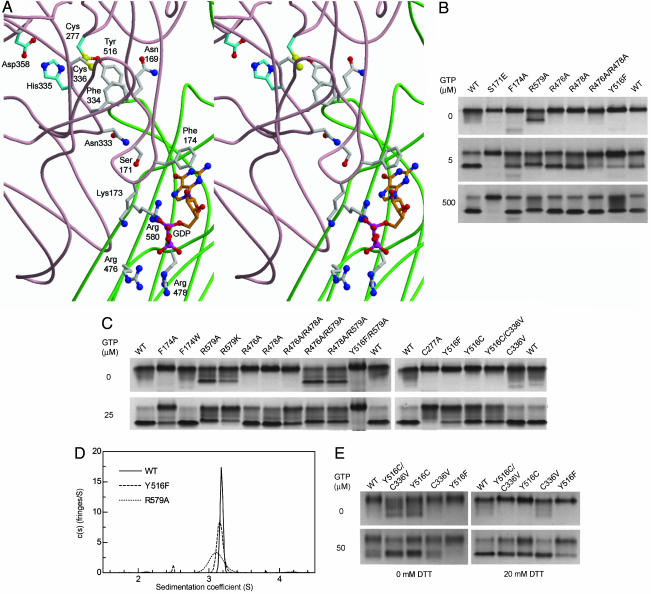
Fig. 1.
Mutations of residues involved in GTP-binding or the Cys-277–Tyr-516 H bond affect TG2 conformation. (A) Model of GDP-bound human TG2 showing the transamidase active center (Cys-277, His-335, and Asp-358) and other key residues [(Protein Data Bank ID code 1KV3) (7); core domain backbone, pale pink; β-barrel 1 domain, green]. Carbon atoms are gray, except those of active site residues (cyan), and GDP (orange). Other atoms include oxygen, red; nitrogen, deep blue; sulfur, yellow; phosphorous, bright pink. Arg-580 in human TG2 is equivalent to Arg-579 in rat TG2. (B and C) Samples (3 μg) of WT and mutant TG2 were incubated (3 h at 25°C) in buffer (50 mM Tris·HCl, pH 7.5/50 mM NaCl/0.5 mM EDTA/1.0 mM MgCl2/5 mM DTT/GTP as indicated). nPAGE was performed with 0.5 mM MgCl2, and the indicated GTP concentrations were included in gel solutions and running buffer. Data are representative of two to eight experiments. (D) Sedimentation coefficient distributions, cM(s), for 13 μM WT (solid line), Y516F (dashed line), and R579A (dotted line) in 10 mM Tris·HCl pH 7.5/100 mM NaCl/0.5 mM EDTA/1 mM DTT/500 μM GTP at 4°C. Molar mass was fixed at 78.0 kDa for 2.5–3.8 S species. Data are representative of three to four experiments. (E) Disulfide trapping of Cys-277 with a cysteine substituted for Tyr-516. Samples (3 μg) of WT and mutant TG2 were oxidized (2 h at 25°C) in buffer (50 mM Tris·HCl, pH 7.5/50 mM NaCl/200:800 μM Cu2+:phenanthroline/50 μM GTP), then incubated (1 h at 25°C) in 0.5 mM MgCl2, without or with 20 mM DTT, as indicated. nPAGE was performed as in B. Data are representative of two experiments.