Abstract
Although Wingless (Wg)/Wnt signaling has been implicated in heart development of multiple organisms, conflicting results have been reported regarding the role of Wnt/β-catenin pathway in cardiac myogenesis: Wg/armadillo signaling promotes heart development in Drosophila, whereas activation of Wnt/β-catenin signaling inhibits heart formation in avians and amphibians. Using an in vitro system of mouse ES cell differentiation into cardiomyocytes, we show here that Wnt/β-catenin signaling exhibits developmental stage-specific, biphasic, and antagonistic effects on cardiomyogenesis and hematopoiesis/vasculogenesis. Activation of the Wnt/β-catenin pathway in the early phase during embryoid body (EB) formation enhances ES cell differentiation into cardiomyocytes while suppressing the differentiation into hematopoietic and vascular cell lineages. In contrast, activation of Wnt/β-catenin signaling in the late phase after EB formation inhibits cardiomyocyte differentiation and enhances the expression of hematopoietic/vascular marker genes through suppression of bone morphogenetic protein signaling. Thus, Wnt/β-catenin signaling exhibits biphasic and antagonistic effects on cardiomyogenesis and hematopoiesis/vasculogenesis, depending on the stage of development.
Keywords: cardiogenesis
Wnt genes encode secreted glycoproteins that play important roles in embryonic development, adult tissue homeostasis, and carcinogenesis (1, 2). During early embryogenesis, Wnt signaling is required for primitive streak formation and mesoderm induction (3, 4). Subsequently, Wnt signals regulate the patterning of anterior-posterior body plan: Wnt signaling is required for trunk/tail development and specification of posterior mesodermal fates, and Wnt inhibition in anterior ectoderm is required for head formation (3, 4). Regarding the role of Wnt signaling in heart formation and cardiomyocyte differentiation, there have been several contradictory reports, depending on the model organisms used. In avians and amphibians, the Wnt/β-catenin pathway inhibits cardiac development, and expression of Wnt inhibitors in the tissue adjacent to cardiac mesoderm is required for cardiogenesis (5–7). In contrast, the Wingless (Wg)/armadillo pathway (which corresponds to the Wnt/β-catenin pathway in vertebrates) promotes heart formation in Drosophila (8, 9), and Wnt/β-catenin signaling induces cardiomyocyte differentiation in a mouse embryonal carcinoma cell line P19CL6 (10, 11). Thus, Wnt/β-catenin signaling inhibits cardiogenesis in chick and Xenopus, whereas it enhances cardiogenesis in flies and in a mouse teratocarcinoma cell line.
To further explore the role of Wnt signaling during cardiac myogenesis, we used mouse ES cells as a model system for cardiomyocyte differentiation in mammals (12). We show here that activation of Wnt/β-catenin pathway in the early phase during embryoid body (EB) formation enhances ES cell differentiation into cardiomyocytes while suppressing hematopoietic and vascular cell marker gene expression. In contrast, activation of Wnt/β-catenin pathway in the late phase inhibits cardiomyocyte differentiation and enhances the expression of hematopoietic/vascular marker genes through the suppression of bone morphogenetic protein (BMP) signaling. Furthermore, initial enhancement followed by inhibition of Wnt/β-catenin signaling results in a marked increase in the efficiency of ES cell differentiation into cardiomyocytes. Thus, Wnt/β-catenin signaling exhibits developmental stage-specific, biphasic, and antagonistic effects on cardiogenesis and hematopoiesis/vasculogenesis, and appropriate modulation of Wnt signaling enables highly efficient cardiomyocyte differentiation of ES cells.
Results
Wnt Signaling Positively Regulates Cardiomyogenesis During Early Stage of ES Cell Differentiation.
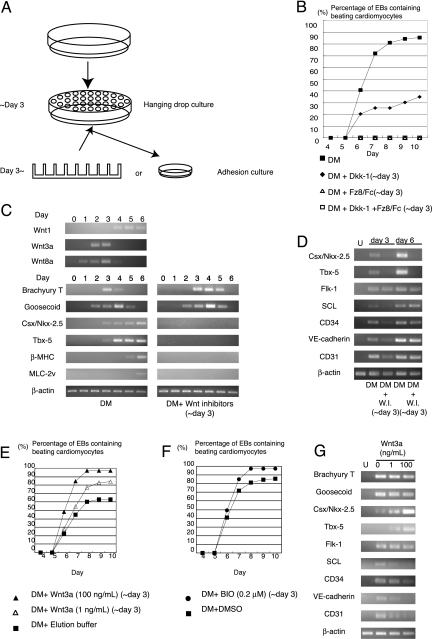
To induce ES cell differentiation into cardiomyocytes, EBs were formed by the hanging-drop method. Hanging-drop culture was started at day 0, and EBs were transferred to adhesion culture on day 3 (Fig. 1A). Under this experimental condition, spontaneous contraction was observed as small contracting foci in ≈80% of EBs at day 8 (Fig. 1B). To investigate the role of Wnt/β-catenin signaling in cardiomyocyte differentiation, we first analyzed the expression of Wnt ligands that activates Wnt/β-catenin pathway such as Wnt1, Wnt3a, and Wnt8a. During ES cell differentiation, Wnt1 was expressed after day 4, Wnt3a on days 2 and 3, and Wnt8a from days 1 to 4 (Fig. 1C). Expression of early mesodermal markers (Brachyury T, Goosecoid) was detected from day 2, and cardiac lineage markers (Csx/Nkx-2.5, Tbx-5) were detectable from day 3 (Fig. 1C). Thus, initial commitment from immature mesodermal cells to a cardiac lineage occurs between days 2 and 3, when Wnt3a and Wnt8a genes are expressed. Next, we blocked Wnt/β-catenin signaling during this early stage of cardiomyocyte differentiation by extracellular Wnt inhibitors, Dickkopf-1 (Dkk-1; 500 ng/ml) and Frizzled-8/Fc chimeric protein (Fz8/Fc) (200 ng/ml), either alone or in combination. At these concentrations, Wnt3a (100 ng/ml)-induced activation of Wnt/β-catenin signaling in ht7 ES cells was completely suppressed as judged by a T cell factor-dependent reporter gene assay and the status of nuclear accumulation of β-catenin [supporting information (SI) Fig. 6]. When ES cells were treated with Dkk-1 in hanging drops from days 0 to 3, EBs were normally formed, but the number of EBs containing spontaneously beating foci was significantly suppressed (Fig. 1B). Moreover, when cells were treated with Fz8/Fc, or Dkk-1 plus Fz8/Fc, spontaneous contraction was never observed up to day 15 (Fig. 1B and data not shown). Although the precise reason for the differential effects of Dkk-1 vs. Fz8/Fc is not known, it may be due to slightly more efficient suppression of Wnt/β-catenin signaling by Fz8/Fc than that by Dkk-1 (SI Fig. 6A). Alternatively, β-catenin-independent, noncanonical Wnt signaling that is blocked by Fz8/Fc but not by Dkk-1 may partially contribute to Wnt-mediated cardiogenesis at this stage. Expression of earliest cardiac marker genes Csx/Nkx-2.5 and Tbx-5 was completely abolished by Wnt inhibitor treatment, whereas that of mesodermal marker genes Brachyury and Goosecoid was not affected by Wnt inhibition (Fig. 1 C and D). Thus, it is presumed that the commitment of mesodermal cells into a cardiomyocyte lineage requires higher levels of Wnt/β-catenin signaling than those required for specification of mesodermal cells. We also investigated whether differentiation into other mesoderm-derived cell lineages is affected by Wnt inhibition. Expression of early hematopoietic markers (SCL and CD34) and endothelial cell markers (CD31 and VE-cadherin) was transiently down-regulated at day 3 but was not altered at day 6. Expression of Flk-1, a marker of hemangioblasts as well as other mesodermal progenitors (13), was not affected by Wnt inhibition (Fig. 1D). Thus, inhibition of Wnt/β-catenin signaling transiently attenuates differentiation of ES cells into a hematopoietic or an endothelial cell lineage. Other mesoderm-derived cell markers such as MyoD (a marker of skeletal muscle) or Runx2 (a marker of bone and cartilage) were not expressed at these time points (data not shown). Taken together, Wnt signaling in the early phase of ES cell differentiation is required for commitment of mesodermal cells into a cardiomyocyte lineage.
Fig. 1.
Wnt/β-catenin signaling positively regulates cardiogenesis in the early stage of ES cell differentiation. (A) Protocol for cardiomyocyte differentiation of ES cells. (B) Number of EBs with beating foci. Wnt inhibitor treatment during EB formation suppressed differentiation into beating cardiomyocytes. (C) RT-PCR analysis of gene expression during differentiation of ES cells into cardiomyocytes. (D) Wnt inhibition blocked expression of cardiac markers and transiently down-regulated hematopoietic/vascular marker genes. Cells were cultured in the presence or absence of Wnt inhibitor during EB formation (until day 3). U, undifferentiated ES cells; W.I., Wnt inhibitors. (E) Number of EBs with beating foci. Wnt3a treatment during EB formation enhanced differentiation of ES cells into beating cardiomyocytes. (F) Number of EBs with beating foci. Treatment with BIO during EB formation enhanced differentiation of ES cells into beating cardiomyocytes. (G) RT-PCR analysis of cardiac, hematopoietic, and vascular cell lineage markers on day 3. U, undifferentiated ES cells.
To further examine the role of Wnt/β-catenin signaling in the commitment of mesodermal cells into cardiomyocytes, we treated ES cells with purified Wnt3a protein during the early stage of ES cell differentiation. When Wnt3a was added from days 0 to 3, the relative number of beating EBs was increased in a dose-dependent manner (Fig. 1E). Slightly lower percentage of spontaneous beating in control EBs as compared with that shown in Fig. 1B is presumably due to the mild inhibitory effect of Wnt3a elution buffer on cardiomyocyte differentiation (Fig. 1 B and E). This effect of Wnt3a to promote cardiogenesis was mimicked by the addition of glycogen synthase kinase-3β inhibitor BIO (0.2 μM) from days 0 to 3, suggesting that β-catenin-dependent canonical Wnt signaling is responsible for this positive effect of Wnt3a on cardiomyocyte differentiation (Fig. 1F). We also examined the expression of cardiac, hematopoietic, and endothelial cell lineage markers in response to Wnt3a treatment at day 3. Addition of Wnt3a from days 0 to 3 dramatically increased the expression of Csx/Nkx-2.5 and Tbx-5, whereas the expression of SCL, CD34, VE-cadherin, and CD31 was decreased in a dose-dependent manner (Fig. 1G and SI Fig. 7). We also performed FACS analysis of hcgp7, a clonal derivative of ht7 ES cells in which GFP cDNA is knocked in at the Csx/Nkx2.5 locus (14). In response to Wnt3a treatment, there was a significant increase in the number of cells in a cardiac lineage (GFP positive) as well as a significant decrease in the number of cells in a hematopoietic (CD34 positive) or an endothelial cell (CD31 positive) lineage at day 3 of differentiation (SI Table 1). Thus, increased level of Wnt/β-catenin signaling during EB formation further enhances differentiation into cardiomyocytes and simultaneously inhibits differentiation of ES cells into hematopoietic and endothelial cell lineages.
Wnt Signaling Negatively Regulates Cardiomyogenesis During the Late Stage of ES Cell Differentiation.
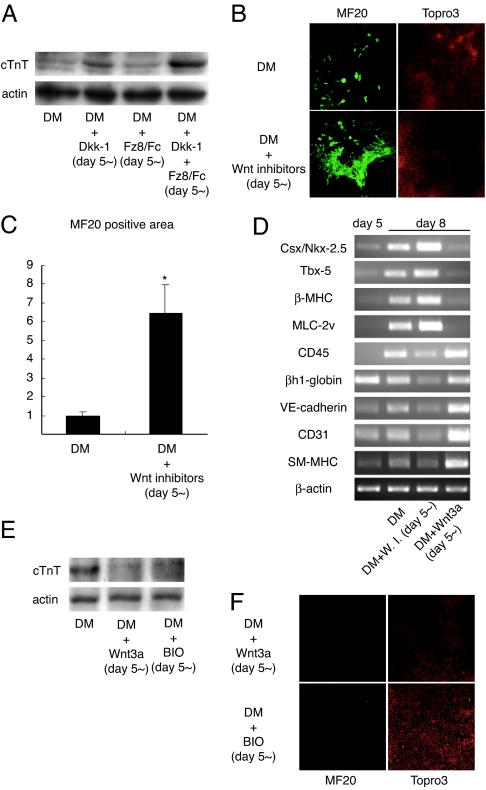
We next examined the role of Wnt/β-catenin signaling during later stage of cardiomyocyte differentiation, when cells are already committed to a cardiac lineage and start to express contractile protein genes. In our experimental condition, the expression of contractile protein genes such as β-myosin heavy chain (β-MHC) and myosin light chain 2v (MLC-2v) was first observed at around day 5 (Fig. 1C). Thus, Wnt inhibitor treatment was started at day 5, and late-stage cardiomyocyte differentiation was evaluated by Western blot analysis of cardiac troponin T (cTnT) on day 10. In contrast to the inhibitory effects of Wnt inhibitors on early-stage cardiomyocyte differentiation, late-stage cardiomyocyte differentiation was strongly promoted by Dkk-1 and only marginally by Fz8/Fc, and a combination of these two inhibitors synergistically enhanced this process (Fig. 2A). This effect is consistent with previous observations showing that Dkk-1 induces ectopic cardiogenesis in noncardiac mesoderm more efficiently than soluble frizzed receptor protein family of Wnt inhibitor such as Frzb (6). In addition, treatment with a combination of Wnt inhibitors (Dkk-1 plus Fz8/Fc) from day 5 increased the area of mature cardiomyocytes stained with MF20 (a monoclonal antibody against sarcomeric MHC) by 6-fold compared with control EBs on day 10 (Fig. 2 B and C). RT-PCR analysis revealed that treatment with Wnt inhibitors increased the expression of mature cardiac marker genes while the expression levels of mature hematopoietic (CD45 and βh1-globin) or endothelial cell markers were decreased (Fig. 2D and SI Fig. 8). Smooth muscle myosin heavy chain (SM-MHC) gene, a marker of smooth muscle cells, was expressed from day 5 during ES cell differentiation (data not shown), and its expression was also decreased by Wnt inhibitor treatment (Fig. 2D and SI Fig. 8). Next, we activated Wnt/β-catenin signaling by treating EBs with Wnt3a or BIO beginning from day 5 of differentiation. In contrast to the effects of Wnt activation on early-stage cardiomyocyte differentiation, activation of Wnt/β-catenin signaling at this later stage completely suppressed the expression of cTnT (Fig. 2E) and abolished the appearance of spontaneously contracting EBs or MF20-positive cardiomyocytes on day 10 (Fig. 2F). The expression of cardiac marker genes was dramatically down-regulated, whereas that of hematopoietic, endothelial, or smooth muscle cell markers was up-regulated by Wnt3a treatment (Fig. 2D and SI Fig. 8). Collectively, these observations indicate that Wnt/β-catenin signaling, when activated after cells are committed to cardiomyocytes, negatively regulates cardiomyocyte differentiation, and that inhibition of Wnt signaling at this stage enhances cardiomyocyte differentiation.
Fig. 2.
Wnt/β-catenin signaling negatively regulates cardiogenesis in the late stage of ES cell differentiation. (A) Western blot analysis for cTnT on day 10. EBs were cultured in DM or DM containing the indicated Wnt inhibitors from day 5. Wnt inhibitor treatment after day 5 increased cTnT expression. (B) MF20 and Topro staining on day 10. (Magnification: ×100.) (C) Morphometry for the expression of sarcomeric MHC in each EB. ∗, P < 0.01 vs. control. (D) RT-PCR analysis of cardiac, hematopoietic, and vascular marker genes. EBs were cultured in DM or DM containing Wnt inhibitors (DM + W.I.) or Wnt3a protein (DM + Wnt3a) from day 5. W.I., Wnt inhibitors. (E) Western blot analysis for cTnT on day 10. EBs were cultured in DM or DM containing Wnt3a (DM + Wnt3a) or BIO (DM + BIO) from day 5. Wnt3a treatment in the late stage of ES cell differentiation blocks further differentiation into mature cardiomyocytes. (F) MF20 and Topro staining on day 10. (Magnification: ×100.)
Activation of Wnt Signaling During the Late Stage Attenuates Cardiomyocyte Differentiation by Suppressing BMP Signaling.
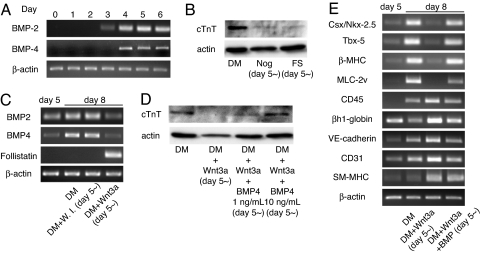
To further investigate the molecular mechanisms by which Wnt/β-catenin signaling shows such biphasic effects on cardiomyocyte differentiation, we investigated whether other signaling pathways that play an important role during cardiogenesis are affected by Wnt/β-catenin signaling. The BMP family of humoral factors positively regulate cardiogenesis in Drosophila (15), chick (16), Xenopus (17), mouse (18), and P19CL6 cells (19). Among the BMP family members, BMP2/4 are implicated in cardiogenesis (16) and are expressed predominantly during the late stage of ES cell differentiation into cardiomyocytes (Fig. 3A). Expression of BMP2/4 during this stage is indispensable for cardiomyocyte differentiation, because the addition of a BMP inhibitor such as noggin (100 ng/ml) or follistatin (100 ng/ml) during the later stage of ES cell differentiation decreased the expression of cTnT (Fig. 3B). Interestingly, activation of Wnt/β-catenin signaling during the late stage of ES cell differentiation suppressed the expression of BMP2/4 and induced the expression of follistatin (Fig. 3C), suggesting that the negative effect of Wnt signaling on cardiomyocyte differentiation in the late phase is mediated by inhibition of BMP pathway. To test this hypothesis, we treated EBs with a combination of Wnt3a and BMP4. Addition of BMP4 protein (1 and 10 ng/ml) rescued the Wnt3a-mediated inhibition of cardiomyocyte differentiation (Fig. 3D) and normalized the expression pattern of cardiac, hematopoietic, and vascular cell marker genes (Fig. 3E). In collection, these observations indicate that the antagonistic effects of Wnt/β-catenin signaling on cardiogenesis and hematopoiesis/vasculogenesis in the late phase of ES cell differentiation are in part mediated by Wnt-induced inhibition of BMP signaling.
Fig. 3.
Wnt inhibits cardiomyocyte differentiation in the late phase by suppressing BMP signaling. (A) RT-PCR analysis of BMP2/4 expression. (B) Western blot analysis for cTnT on day 10. EBs were cultured in DM or DM containing noggin (Nog) or follistatin (FS) from day 5. Inhibition of BMP signaling during the late phase suppresses cardiomyocyte differentiation. (C) RT-PCR analysis of BMP2/4 and Follistatin expression. EBs were cultured in DM or DM containing Wnt inhibitors (DM + Wnt inhibitors) or Wnt3a protein (DM + Wnt3a) from day 5. W.I., Wnt inhibitors. (D) Western blot analysis for cTnT on day 10. EBs were cultured in DM or DM containing Wnt3a (DM + Wnt3a) or Wnt3a plus BMP4 protein (DM + Wnt3a + BMP4) from day 5. Inhibition of cardiomyocyte differentiation by Wnt3a was rescued by BMP treatment. (E) RT-PCR analysis of cardiac, hematopoietic, and vascular marker genes. EBs were cultured in DM or DM containing Wnt3a (DM + Wnt3a) or Wnt3a plus BMP4 protein (DM + Wnt3a + BMP4) from day 5.
Modulation of Wnt Signaling Enables Efficient Induction of Cardiomyocytes from ES Cells.
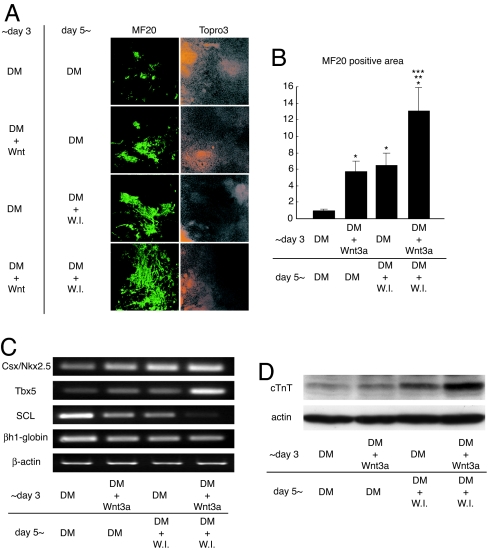
Finally, we tested the hypothesis that activation of Wnt/β-catenin signaling in the early phase (from days 0 to 3) followed by Wnt inhibition in the late phase (from day 5 and afterward) might further enhance cardiomyocyte differentiation of ES cells. Activation of Wnt signaling in the early phase or inhibition of Wnt signaling in the late phase each increased MF20-positive area ≈6-fold (Fig. 4A and B). Wnt activation in the early phase followed by Wnt inhibition in the late phase further increased MF20-positive area per single EB up to 13-fold (Fig. 4 A and B). This increase in MF20-positive area was associated with increased expression of Csx/Nkx2.5 and Tbx5 and decreased expression of SCL and βh1-globin (Fig. 4C; SI Fig. 9). Enhanced cardiomyocyte differentiation and diminished hematopoietic differentiation was also confirmed by Western blot analysis of cTnT expression and FACS analysis of hcgp7 cells on day 10 of differentiation (Fig. 4D; SI Table 2). Thus, appropriate modulation of Wnt signaling enables highly efficient induction of ES cell differentiation into cardiomyocytes.
Fig. 4.
Activation of Wnt signaling in the early stage followed by Wnt inhibition in the late stage leads to highly efficient differentiation of ES cells into cardiomyocytes. (A) MF20 and Topro staining on day 10. (Magnification: ×100.) (B) Morphometry for the expression of sarcomeric MHC in each EB. ∗, P < 0.05 vs. “DM>DM” protocol. ∗∗, P < 0.05 vs. “DM + Wnt3a>DM” protocol. ∗∗∗, P < 0.05 vs. “DM>DM + Wnt inhibitor” protocol. (C) RT-PCR analysis of cardiac and hematopoietic markers. (D) Western blot analysis of cTnT expression on day 10.
Discussion
Although previous studies have implicated Wg/Wnt signaling in cardiac development, there exist some controversies regarding the roles of Wg/Wnt in cardiogenesis. In Drosophila, elimination of Wg function for a short time period after gastrulation results in loss of heart formation, and overexpression of Dishevelled leads to increased number of heart precursor cells (8, 9). Likewise, Wnt/β-catenin signals are essential for in vitro cardiomyocyte differentiation of mouse P19CL6 cells, a teratocarcinoma-derived pluripotent cell line (10, 11). However, in chick embryos at stage 5–6, Wnt3 and Wnt8 are expressed in the posterior part of the embryo (primitive streak and adjacent ectodermal cells) and block cardiogenesis in this region (5). At stage 8–9, Wnt1 and Wnt3 are expressed in the neural tube, and this Wnt signal from neural tube blocks cardiogenesis in the anterior paraxial mesoderm (7). In Xenopus embryos, forced expression of Wnt ligands in dorsal mesoderm (which normally gives rise to the heart) inhibits cardiac marker gene expression (6). Taken together, the Wnt/β-catenin pathway promotes cardiogenesis in Drosophila and mouse P19CL6 cells, whereas it blocks heart formation in chick and Xenopus.
Based on our present study, one possible explanation for this apparent discrepancy is that Wnt/β-catenin signaling has biphasic effects on cardiogenesis depending on the stage of differentiation. According to this model, Wnt signaling promotes commitment of mesodermal cells into a cardiac lineage in the early phase of cardiogenesis while it inhibits proliferation and/or maturation of committed cardiomyocytes in the late phase of cardiac development. In Drosophila, temporal requirement of Wg for heart formation is restricted to a short time period after gastrulation, which is much earlier than the time point when the expression of a Csx/Nkx-2.5-related gene tinman becomes restricted to dorsal cardiac mesoderm (8). During P19CL6 cell differentiation into cardiomyocytes, expression of Wnt3a and Wnt8a genes is observed earlier than that of early cardiac markers, and inhibition of Wnt signaling in the early stage results in the suppression of cardiac marker gene expression without affecting mesodermal marker gene expression (10, 11). These observations support the notion that Wnt/β-catenin signaling promotes commitment of mesodermal cells into a cardiomyocyte lineage. In chick and Xenopus, negative effects of Wnt signals on cardiogenesis are observed in precardiac mesoderm, where cells are supposed to be already committed to a cardiomyocyte lineage. In support of this hypothesis, it was reported that hemangioblast commitment is already initiated during gastrulation in the primitive streak (20). By analogy, the cell fate decision into a cardiac lineage may occur during gastrulation in the primitive streak, where a high concentration of local Wnt ligands promotes this process, and then committed cells migrate to heart forming region by gastrulation movements where they are exposed to Wnt inhibitors to form mature cardiomyocytes.
Wnt/β-catenin signaling is required for primitive streak formation and mesoderm induction in vivo (3, 4) and the generation of ES cell-derived mesoderm in vitro (21). Thus, one might argue that the positive effects of Wnts on cardiogenesis in the early stage of differentiation reflect the mesoderm-inducing activity of Wnt signaling. However, we favor the model in which additional Wnt activity is required to induce commitment of immature mesodermal cells into a cardiac lineage, because Wnt inhibitor treatment in the early phase (from days 0 to 3) did not attenuate the expression of mesodermal markers while completely abolishing early cardiac marker gene expression in our experimental condition. The discrepancy between the present work and a previous study (21) may be due to a difference in the ES cell differentiation protocol. Alternatively, the concentration of Wnt inhibitors used in our study might be subthreshold level to completely block mesodermal differentiation (Fig. 1C). During gastrulation, heart progenitor cells leave the primitive streak and move anterolaterally to reside at the anterior end of the mesoderm, making the heart the most anterior mesoderm-derived organ to develop in vertebrates. In this regard, the requirement of Wnt inhibitors for cardiogenesis in the later stage of ES cell differentiation may reflect the regulation of anterior-posterior body plan by Wnt signaling.
Another important point is that Wnt signal has antagonistic effects on cardiogenesis vs. hematopoiesis/vasculogenesis in both the early and late stages of differentiation. Wnt/β-catenin signaling was initially proposed to increase hematopoietic stem cell (HSC) self-renewal and enhance their ability to reconstitute the hematopoietic system of lethally irradiated animals (2). On the other hand, constitutive activation of Wnt/β-catenin signaling in hematopoietic system results in differentiation block of HSCs and widespread hematopoietic abnormalities (22, 23), together suggesting that fine-tuned control of Wnt signaling is required for HSC self renewal and differentiation. This notion is consistent with our observations that both activation and inhibition of Wnt signaling in the early phase of ES cell differentiation result in down-regulation of hematopoietic marker genes. Regarding the antagonistic effects of Wnt signaling on cardiogenesis and hematopoiesis in the late phase of ES cell differentiation, similar effects of Wnt signaling on cardiogenesis vs. hematopoiesis have been reported in chick and frog embryos: Wnt inhibition in posterior mesoderm (which normally gives rise to blood) leads to induction of cardiac markers and simultaneous inhibition of hematopoietic marker gene expression (5), and forced expression of Wnt ligands in precardiac mesoderm leads to inhibition of cardiogenesis and ectopic expression of hematopoietic markers (5, 6).
We have also shown that Wnt-induced inhibition of cardiogenesis and promotion of hematopoiesis/vasculogenesis in the late phase are in part mediated by Wnt-induced inhibition of BMP signaling. This notion is consistent with a recent report showing that inhibition of BMP signaling enhances hematovascular development in lateral mesoderm in zebrafish (24). Wnt-induced BMP inhibition is also reported by previous studies showing that Wnt/β-catenin signaling attenuates the expression of BMP4 during neurogenesis (25) and promotes the expression of follistatin in mouse embryonal carcinoma cells (26). However, it should be noted that Wnt-mediated inhibition of BMP signal is context-dependent, because Wnt3a induces the expression of BMP4 in a different cell type (27).
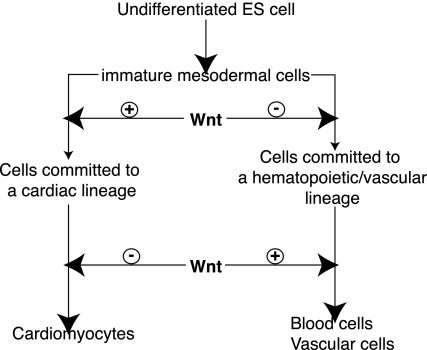
Collectively, our present study indicates that Wnt signals have developmental stage-specific, biphasic, and antagonistic effects on both cardiogenesis and hematopoiesis/vasculogenesis (Fig. 5) and provides important clues to the dissection of cardiogenic signaling pathways during embryogenesis. It also demonstrates that differentiating ES cells represent a useful model system for the dissection of complex regulatory networks that control organogenesis during embryonic development.
Fig. 5.
Wnt signals exhibit developmental stage-specific, biphasic, and antagonistic effects on cardiogenesis and hematopoiesis. In the early stage of development, Wnt signals promote cardiogenesis and inhibit hematopoiesis, whereas in the late stage of development, Wnt signals inhibit cardiomyocyte differentiation and promote blood cell differentiation.
Materials and Methods
Reagents.
Recombinant mouse Dkk-1, mouse Fz8/Fc, mouse noggin/Fc chimera, mouse Follistatin 288, and human BMP4 proteins were purchased from R&D (Minneapolis, MN). The glycogen synthase kinase-3β inhibitor, BIO, was from Calbiochem (La Jolla, CA). Wnt3a protein was purified as described (28). Elution buffer for Wnt3a protein contains 1% CHAPS and 30 mM imidazole. Same amount of elution buffer was added to the culture medium when using purified Wnt3a protein.
ES Cell Culture and Differentiation Protocol.
The 129/Ola-derived ht7 ES cells (29) and hcgp7 ES cells (14) were used in this study. ES cells were maintained on gelatin-coated dishes without feeder cells by using growth medium containing 2,000 units/ml leukemia inhibitory factor (ESGRO; Chemicon, Hampshire, U.K.). For differentiation, 500 ES cells in 30-μl aliquots of (DM; growth medium without leukemia inhibitory factor) were cultured in hanging drop for 3 days. On the third day, the resultant individual EBs were transferred to gelatin-coated 48-well culture plates or a 35-mm dish. To evaluate the differentiation efficiency of ES cells, 48-well plates were monitored every day under a microscope to detect the appearance of spontaneously contracting cardiomyocytes, and the percentage of the EBs that exhibited spontaneous contraction was calculated as differentiation efficiency. More than 200 wells were observed to calculate differentiation efficiency at each time point. The medium was changed every other day. The day when hanging drop culture was started was defined as day 0.
RT-PCR and Quantitative Real-Time PCR.
Total RNA extraction and DNase treatment were performed by using the SV total RNA isolation Kit (Promega, Madison, WI). RT-PCR was performed as described (10). Every PCR condition was confirmed to be within the semiquantitative range for specific genes and primer pairs. Expression of β-actin was used as internal control. Real-time PCR was performed by using the LightCycler (Roche, Indianapolis, IN) according to the manufacturer's instructions. Individual PCR products were analyzed by melting-point analysis. β-Actin was used as an internal control to normalize for RNA amounts. Relative levels of gene expression were normalized to the β-actin gene by using the comparative Ct method according to the manufacturer's instructions. Primer sequences for both semiquantitative and quantitative PCR are available upon request.
Western Blotting.
Western blotting was performed as described (10). Signal was detected by using ECL detection kit (Amersham Biosciences, Piscataway, NJ).
Immunohistochemistry.
Immunohistochemistry was performed as described (30). Mouse monoclonal antisarcomeric myosin heavy chain (MF20; Developmental Studies Hybridoma Bank maintained at the University of Iowa, Department of Biological Sciences, Iowa City, IA) was used as a primary antibody, and FITC-conjugated secondary antibody was applied to visualize expression of specific proteins. Before mounting, nuclei were stained with Topro3 (Molecular Probes, Eugene, OR). Images of samples were taken by laser confocal microscopy (Radiance 2000; Bio-Rad Laboratories, Hercules, CA). For morphometric analysis for the expression of sarcomeric myosin heavy chain stained with MF20, all aspects of cell processing, immunostaining, and imaging were rigorously standardized. To exclude the possibility that variations in immunostaining on different samples affected the morphometric data, all samples in the same data set were immunostained and analyzed at the same time. Digital images were obtained from at least four EBs using the ×10 objective lens. Four images from at least three independent immunostained samples were used for morphometric analysis. The MF20-positive area was calculated as fold increase to that of control EB induced to differentiate into cardiomyocytes with no extra treatment.
FACS Analysis.
FACS was performed by using EPICS ALTRA (Beckman Coulter, Fullerton, CA). Cells were dissociated by using 0.05% trypsin (Invitrogen) in PBS and stained with phycoerythrin (PE)-conjugated anti-CD31, -CD34, or -CD45 (all from e-bioscience, San Diego, CA) for 30 min on ice and washed twice with PBS supplemented with 2% FBS. PE and GFP were detected by using a 488-nm argon laser.
Statistical Analysis.
Data are expressed as mean ± standard deviation. The significance of differences among means was evaluated by using ANOVA, followed by Fisher's probable least-squares difference test for multiple comparisons. Significant differences were defined as P < 0.05.
Supplementary Material
Acknowledgments
We thank Hitoshi Niwa (Osaka University, Osaka, Japan) for the ht7 ES cells. This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports, and Culture; the Japan Health Sciences Foundation; Health and Labor Sciences; and the Japan Medical Association (to I.K.); and by a Japan Heart Foundation Young Investigator's Research Grant (to A.T.N.).
Abbreviations
- BMP
bone morphogenetic protein
- cTnT
cardiac troponin T
- Dkk-1
Dickkopf-1
- EB
embryoid body
- Fz8/Fc
Frizzled-8/Fc chimeric protein
- Wg
Wingless
- DM
differentiation medium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605768103/DC1.
References
- 1.Logan CY, Nusse R. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Clevers H. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi TP. Curr Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- 4.Kimelman D. Nat Rev Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- 5.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider VA, Mercola M. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzahor E, Lassar AB. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Golden K, Bodmer R. Dev Biol. 1995;169:619–628. doi: 10.1006/dbio.1995.1174. [DOI] [PubMed] [Google Scholar]
- 9.Park M, Wu X, Golden K, Axelrod JD, Bodmer R. Dev Biol. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- 10.Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Sano M, Songyang Z, Schneider MD. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller G. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 13.Ema M, Takahashi S, Rossant J. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka K, Lee JK, Kim HS, Ihm CH, Iio A, Ogawa M, Nishikawa S, Kodama I, Morisaki T. FASEB J. 2003;17:740–742. doi: 10.1096/fj.02-0104fje. [DOI] [PubMed] [Google Scholar]
- 15.Frasch M. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- 16.Schultheiss TM, Burch JB, Lassar AB. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Katsev S, Cai C, Evans S. Dev Biol. 2000;224:226–237. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Bradley A. Development (Cambridge, UK) 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 19.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, et al. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 21.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Development (Cambridge, UK) 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 22.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 23.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Zhu H, Zon LI, Evans T. Development (Cambridge, UK) 2006;133:2177–2187. doi: 10.1242/dev.02386. [DOI] [PubMed] [Google Scholar]
- 25.Baker JC, Beddington RS, Harland RM. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willert J, Epping M, Pollack JR, Brown PO, Nusse R. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler DG, Sutherland MS, Ojala E, Turcott E, Geoghegan JC, Shpektor D, Skonier JE, Yu C, Latham JA. J Biol Chem. 2005;280:2498–2502. doi: 10.1074/jbc.M400524200. [DOI] [PubMed] [Google Scholar]
- 28.Kishida S, Yamamoto H, Kikuchi A. Mol Cell Biol. 2004;24:4487–4501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa H, Miyazaki J, Smith AG. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 30.Naito AT, Tominaga A, Oyamada M, Oyamada Y, Shiraishi I, Monzen K, Komuro I, Takamatsu T. Exp Cell Res. 2003;291:56–69. doi: 10.1016/s0014-4827(03)00378-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.