We read with interest the recent commentary by Friedrich and colleagues [1] that reported a meta-analysis of the two placebo-controlled clinical trials of Drotrecogin alfa (activated) (Drot Aa): the PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation of Severe Sepsis) [2] and ADDRESS (Administration of Drotrecogin Alfa [Activated] in Early Stage Severe Sepsis) [3] trials. Several methodological concerns in the analysis by Friedrich and colleagues severely limit the utility of the analysis and call into question the conclusions.
In their meta-analysis, the authors pooled all data from PROWESS and ADDRESS and conclude that there is no statistically significant treatment effect associated with Drot Aa in the overall population and in subgroups of patients with Acute Physiology And Chronic Health Evaluation (APACHE) II score ≥ 25 or multiple organ dysfunction (MOD). Rather than relying on combining point estimates of pooled results, we performed a more straightforward and standard meta-analysis for the MOD populations by combining patient level data to fit a logistic regression model with terms for treatment, study and their interaction (a fixed effect approach). This model yields a significant common treatment effect estimate with p = 0.01. Given that the study interaction was non-significant, we combined MOD data in an unadjusted analysis that yielded a significant common treatment effect estimate with p = 0.007.
To further explore the authors' methods, we utilized the same standard meta-analysis software (Review Manager, version 4.2) and entered the mortality results for patients with APACHE II score ≥ 25 from PROWESS and ADDRESS [4]. For dichotomous data, such as mortality, the software provides meta-analysis methods using a random effect model as provided by Friedrich and colleagues, but also fixed effect models (Mantel-Haenzel risk ratio and Peto odds ratio). As illustrated in Figure 1, the results from the fixed effect models differ substantially from the random effect model by using greater weights for PROWESS. These weights are closer to those seen when the data are simply combined, where PROWESS contributed 72% of the patients and 78% of the mortality. The authors fail to provide their rationale for choosing the random effect model versus the fixed effect model provided by this software. Thus, we believe that only providing results from the random effect model meta-analysis is a significant flaw in Friedrich and colleagues approach. We conclude that these more appropriate types of meta-analyses demonstrate that the treatment effect of Drot Aa in the indicated population of severe sepsis patients is, in fact, robust.
Figure 1.
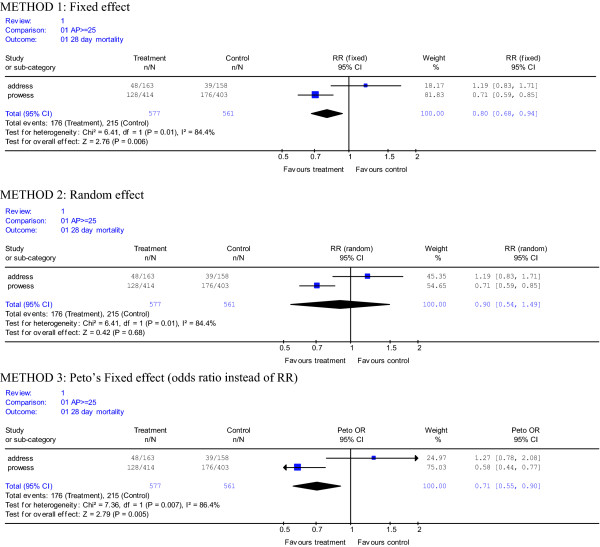
Meta-analysis of PROWESS and ADDRESS patients with APACHE II score (AP) ≥ 25 using output from standard meta-analysis software (Review Manager, Version 4.2). The results from the following three methods are presented: method 1, a fixed effect model demonstrating a risk ratio using weights for ADDRESS and PROWESS of 18.17% and 81.83%, respectively; method 2, a random effect model demonstrating a risk ratio using weights for ADDRESS and PROWESS of 45.35% and 54.65%, respectively; and method 3, a fixed effect model demonstrating a Peto odds ratio using weights for ADDRESS and PROWESS of 24.97% and 75.03%, respectively. The risk ratios (RR) and odds ratio (OR) are plotted on a natural logarithm scale. n/N equals number of deaths at 28 days/number of patients for each particular group. Each method has a Chi-square test for heterogeneity, degrees of freedom (df) that is 1 less than the number of studies and a p value < 0.05, indicating heterogeneity. A point estimate to the left of the line of identity (1) favors treatment with Drotrecogin alfa (activated) and an estimate to the right favors placebo. A 95% confidence interval (CI) is included for each point estimate.
We strongly believe that repeating a placebo-controlled trial with Drot Aa in patients deemed to be at high-risk of death is not only unethical, but also not practical with a drug already approved by regulatory bodies to improve survival in this fragile population. Even if such a trial were deemed ethical because of clinical equipoise, the selection biases introduced into any such study would be substantial and would reduce the chance for any meaningful outcome. Importantly, regulatory bodies have fully reviewed the ADDRESS study data and have concluded that the results do not undermine the risk benefit profile of Drot Aa established in PROWESS, upon which approval is based.
Abbreviations
APACHE = Acute Physiology And Chronic Health Evaluation; Drot Aa = Drotrecogin alfa (activated); MOD = multiple organ dysfunction.
Competing interests
Dr Williams, Dr Janes and Mr Nelson are all employees and stockholders in Eli Lilly and Company, the manufacturer of Xigris [Drot Aa].
See related commentary by Friedrich et al., http://ccforum.com/content/10/3/145
Contributor Information
Mark D Williams, Email: mardwill@lilly.com.
Jonathan M Janes, Email: jonathan.janes@lilly.com.
David R Nelson, Email: nelsondr@lilly.com.
References
- Friedrich JO, Adhikari NKJ, Meade MO. Drotrecogin alfa (activated): does current evidence support treatment for any patients with severe sepsis? Crit Care. 2006;10:145. doi: 10.1186/cc4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand D, Ely EW, for the Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. New Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, for the Administration of Drotrecogin alfa (activated) in Early Stage Severe Sepsis (ADDRESS) Study Group et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. New Engl J Med. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- Review Manager http://www.cochrane-net.org


