Abstract
Recently, a population of nerves has been described in the aganglionic mouse vas deferens, in which electrically evoked contractions were insensitive to high concentrations of the adrenergic neurone blocker, bretylium. In this paper, the pharmacology of this nerve-evoked contraction has been examined in more detail.
Bretylium (20 μM) revealed, after 5 h exposure, a new residual neurogenic contraction (20 stimuli at 10 Hz) that was tetrodotoxin-sensitive.
The muscarinic antagonist, cyclopentolate (0.1 and 1 μM), reduced this residual component and the inhibition was reversed by the acetylcholinesterase inhibitor, neostigmine (1 and 10 μM).
Nicotine (30 μM) enhanced the residual component revealed by bretylium, suggesting that there are prejunctional nicotinic receptors (nAchRs) influencing acetylcholine (Ach) release.
In the presence of prazosin (0.1 μM), a selective α1-adrenoceptor antagonist, and α,β-methylene ATP (1 μM), a purinergic agonist that desensitise P2X receptors, neostigmine increased the hump component of contraction and yohimbine (0.3 μM), an α2-adrenoceptor antagonist, enhanced both components of the electrically evoked stimulation. The contraction was blocked by cyclopentolate (1 μM).
In the absence of bretylium, neostigmine alone increased the hump component of contraction in a frequency-dependent manner. This increase was reversed by atropine (1 μM) and cyclopentolate (1 μM) to control levels. However, in control experiments, atropine or cyclopentolate did not detectably influence the delayed neurogenic contraction.
Ach (10 μM) induced a contraction in the mouse vas deferens, either when applied alone or in the presence of neostigmine.
Thus, it has been demonstrated unequivocally that the mouse vas deferens is innervated by functional cholinergic nerves, whose action is terminated by cholinesterase. Furthermore, Ach release can be enhanced by activation of prejunctional nAchRs presumably located on the cholinergic nerve terminals.
Keywords: Mouse vas deferens, contraction studies, bretylium, neostigmine, acetylcholine
Introduction
It is known that the mouse vas deferens receives a dense sympathetic innervation (see Morris & Gibbins, 1992), organ bath studies revealing the classical biphasic neurogenic contraction composed of two phases known as the ‘twitch' (fast, purinergic) and a delayed, better maintained second component the ‘hump' (slow, noradrenergic).
However, recently we reported a neurogenic cholinergic component of contraction (Jackson & Cunnane, 2002): the cholinergic component was revealed as a bretylium-resistant delayed contraction, which was blocked by atropine.
Other research groups have previously reported a cholinergic component of contraction in the vas deferens of various species (e.g. rat, guinea-pig, cat, rabbit) (Kujat et al., 1964; Dixon & Gosling, 1972; Miranda et al., 1988). Furthermore, immunohistochemical studies have demonstrated the presence of a cholinergic innervation, largely confined to the circular smooth muscle layer (Majcen, 1984). Indeed, Ventura et al. (1998) have reported nitric oxide synthase and vasointestinal peptide (VIP) labelling of cholinergic fibres in rat vas deferens and a cholinergic component of the neurogenic contraction. Cholinergic nerves come from the pelvic ganglia, which are mixed sympathetic–parasympathetic ganglia, and provide the majority of the autonomic innervation to the urogenital organs (Wanigasekara et al., 2003). In addition, the vas deferens is also supplied with sensory nerves (Hökfelt & Ljungdahl, 1972; Saito et al., 1987; Geppetti et al., 1988). Drugs that destroy sympathetic nerves, such as guanethidine, deplete the vas deferens of noradrenaline (NA) and neuropeptide Y, while levels of VIP and calcitonin gene-related peptide (CGRP) are increased (Aberdeen et al., 1990). Thus, it is clear that the vas deferens is innervated by cholinergic and sensory nerve fibres, as well as the classical innervation by ‘short' intact sympathetic neurones.
Activation of prejunctional nicotinic receptors (nAchRs) has been shown to facilitate neurotransmitter release in the aganglionic vas deferens (Starke et al., 1991; Von Kugelgen & Starke, 1991a, 1991b; Carneiro et al., 1993; Markus et al., 1996; Zago & Markus, 1999) and Ca2+ imaging techniques have demonstrated that all varicose nerve terminals in the mouse vas deferens are excited by nicotine and related agonists (Brain et al., 2001). However, in many strings of varicosities, the adrenergic neurone blocker, bretylium, failed to block action potential-evoked Ca2+ transients (Jackson & Cunnane, 2002). These strings were considered to be preterminal sympathetic fibres, sensory nerves or cholinergic nerves.
In the present study, the characteristic features of the neurogenic cholinergic component of contraction of the mouse vas deferens have been further investigated. The aims were to characterise pharmacologically the cholinergic component of contraction and to determine whether prejunctional nAchRs could modulate Ach release.
Methods
Male Balb/C mice (8–12 weeks, 30–50 g) were humanely killed, according to the guidelines of the U.K. Animal (Scientific Procedures) Act 1986, by concussion and cervical dislocation. The vasa deferentia were excised and placed in physiological saline, gassed with 95% O2/5% CO2 to pH 7.4 and maintained at 37°C. The composition of the physiological saline was (mM): NaCl 118.8, NaHCO2 25, NaH2PO4 1.13, KCl 4.7, CaCl2 1.8, MgCl2 1.2 and glucose 11.1.
Contraction studies
In all experiments, the prostatic third of each vas deferens was removed to produce an aganglionic preparation (to ensure that there is no ganglion) knowing the fact that the hypogastric ganglion is located in the proximity of the prostatic end. The surrounding connective tissue was also removed. Each vas deferens was mounted in either a 5 or 25 ml organ bath (according to the experiment) and connected to an isometric transducer (Letica Scientific Instruments, Spain), under an initial tension of 9.8 mN, to record contraction of the longitudinal smooth muscle layer. Contraction data were digitised at a sampling rate of 10 Hz, using a MacLab/8 s data acquisition system, Chart v.3.6.8/s software (AD Instruments, U.K.) and data stored on a Macintosh computer. The maximum amplitude was the criteria for analysing the contractions. Preparations were allowed to equilibrate for 60 min before controls were recorded, tissues being washed with fresh physiological saline every 20 min throughout each experiment. Electrical field stimuli were applied through platinum double ring electrodes (diameter 2 mm, separation 3 mm), connected to a digital stimulator built in-house. Two different experimental protocols were used. In bretylium experiments, trains of 5 and 200 stimuli were delivered every 30 min (0.5 ms duration, 10 Hz, supramaximal voltage). Trains of 5, 50, 100 and 200 pulses (using the same parameters) were given every 20 and 60 min in experiments in which bretylium was not used. An interval of 5 min was left between each train. Contractions evoked by these stimulation protocols were abolished by tetrodoxin (TTX, 0.3 μM, n=6).
Drugs
Stock solutions of TTX, α,β-methylene adenosine triphosphate (ATP) lithium salt, atropine sulphate, cyclopentolate hydrochloride, neostigmine bromide, bretylium tosylate, yohimbine hydrochloride and nicotine hydrogen tartrate were prepared in distilled water. Prazosin hydrochloride was dissolved in dimethyl sulphoxide. Solutions were aliquoted and stored at −20°C, ensuring that the drugs only passed through one freeze–thaw cycle. The drugs were serially diluted in Krebs to the required final concentration on the day of use. Vehicle controls had no effects. All compounds were obtained from Sigma (Dorset, U.K.).
Analysis
Data are expressed as the mean±s.e.m.; n represents the number of vas deferens per experiment. Statistical significance was determined using a paired Student's t-test. Asterisks seen in the figures represent P<0.05.
Results
Effects of bretylium on neurogenic contractions of the mouse vas deferens
The adrenergic neurone blocker bretylium (20 μM, Figure 1) greatly reduced or abolished both the purinergic and noradrenergic components of contraction in mouse isolated vas deferens. Preparations were stimulated every 30 min with trains of 200 stimuli at 10 Hz. Neurogenic contractions were recorded before and during prolonged (up to 5 h) exposure to bretylium. A residual, low-amplitude biphasic contraction was slowly revealed after approximately 3 h exposure to bretylium. Interestingly, this contraction appeared to increase in amplitude over the time period of exposure to bretylium, typically reaching a maximum after 5 h (see Jackson & Cunnane, 2002). Also, the amplitude of this component of contraction seemed to depend on the age of the mice. In 8-week-old mice (young mice), the bretylium-resistant component was not present or was of low amplitude (mean amplitude of the slow component 1.2±0.2 mN, n=6). In 12-week-old mice (old mice), the component was observed, usually after 3–5 h exposure of preparations to bretylium (mean amplitude of the slow component 1.9±1.0 mN, n=5).
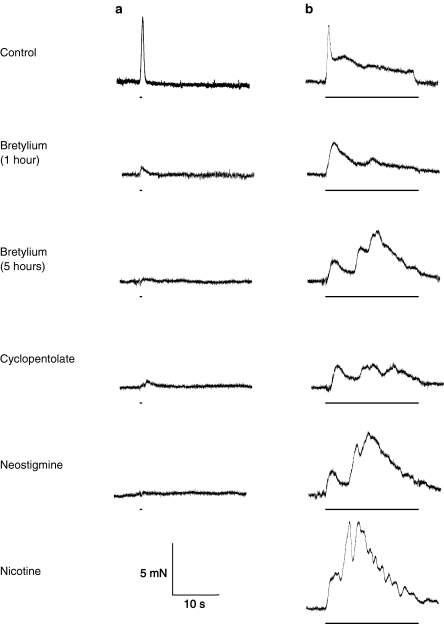
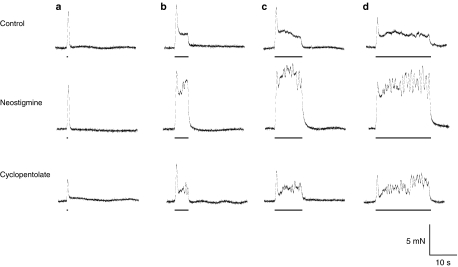
Figure 1.
Effect of cyclopentolate and neostigmine on the bretylium-resistant contraction. Typical traces showing electrically evoked contractions evoked by trains of 5 (a) and 200 (b) stimuli at 10 Hz in 12-week-old mouse vas deferens. In the presence of bretylium (20 μM), contraction was reduced (after 1 h) and, after 5 h, a residual component was revealed. Cyclopentolate (1 μM) reduced this residual component, an effect reversed by neostigmine (10 μM). The addition of nicotine (30 μM) increased this residual component. The bars represent the periods of electrical stimulation.
Effects of cyclopentolate on the bretylium-resistant component of contraction
The competitive muscarinic receptor (mAchR) antagonist cyclopentolate (1 μM) reduced the amplitude of the bretylium-resistant component of contraction (Figure 1; mean amplitude of the slow component 0.5±0.1 mN, n=6, P<0.05 in young mice and mean amplitude of the slow component 0.6±0.1 mN, n=5, P<0.05 in old mice, respectively).
Effects of neostigmine in the presence of bretylium
Neostigmine (10 μM) increased the amplitude of the second component of the bretylium-resistant contraction, in the presence (Figure 1; data for 10 μM neostigmine, mean amplitude of the fast component 1.4±0.2 mN; mean amplitude of the slow component 5.4±0.8 mN, n=9, P<0.05) or the absence of cyclopentolate (mean amplitude of the fast component 1.2±0.4 mN; mean amplitude of the slow component 5.8±0.5 mN, n=5, P<0.05). Since Ach degradation is blocked by neostigmine, the cyclopentolate-induced competitive blockade of mAchRs was likely overcome. Furthermore, neostigmine revealed a large cholinergic component of contraction in the vas deferens, later in young mice, which was undetectable in bretylium-treated controls (mean amplitude of the fast component 1.2±0.2 mN; mean amplitude of the slow component 7.0±0.5 mN, n=6, P<0.05).
Effects of nicotine on the bretylium-resistant component of contraction in the presence and absence of cyclopentolate and neostigmine
Nicotine (30 μM) further increased the residual cholinergic neurogenic contraction in the presence of neostigmine in both young (n=6) and old (n=5) mice (mean amplitude of the fast component 3.4±1.8 and 1.7±0.6 mN; mean amplitude of the slow component 7.7±0.8 and 7.4±0.6 mN). In the presence of both cyclopentolate and neostigmine, nicotine potentiated the neurogenic contraction only in six out of nine preparations, using old mice (mean amplitude of the fast component 1.6±0.5 mN; mean amplitude of the slow component 7.4±1.4 mN, n=9).
It is noteworthy that an early, monophasic contraction replaced the initial ‘twitch' contraction after bretylium treatment (see Figure 1). This unexpected TTX-sensitive contraction has not been further investigated in the present study other than to say that it was insensitive to cyclopentolate and neostigmine but potentiated by nicotine.
Effects of neostigmine, yohimbine and cyclopentolate in the presence of prazosin and α,β-methylene ATP
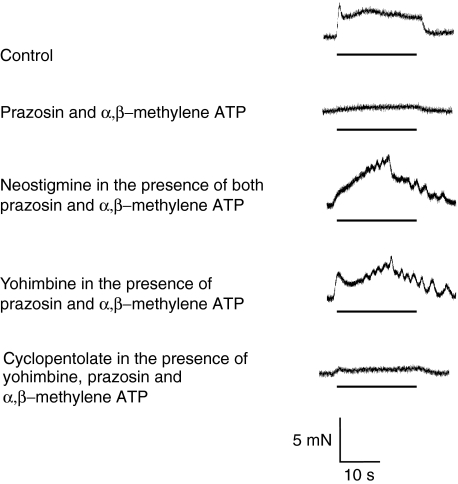
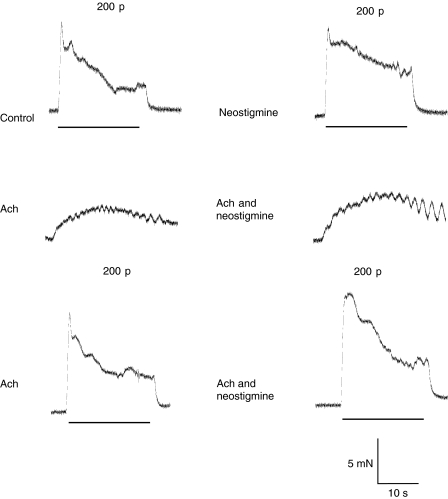
Bath application of prazosin (100 nM) and α,β-methylene ATP (1 μM) inhibited the sympathetic response in the mouse vas deferens within 30–40 min, but a small residual component of contraction was still detected (Figure 2, n=6). The amplitude of this component was increased by neostigmine (Figure 3; 10 μM, P<0.05) and yohimbine (Figure 3; 0.3 μM), and the increase was inhibited by cyclopentolate (Figure 3; 1 μM).
Figure 2.
Effects of combinations of prazosin, α,β-methylene ATP, neostigmine, yohimbine and cyclopentolate on neurogenic contractions of the mouse vas deferens. Typical traces showing electrically evoked contractions evoked by trains of 200 stimuli (10 Hz). In the presence of prazosin (0.1 μM) and α,β-methylene ATP (1 μM), the contraction was markedly reduced. Neostigmine (10 μM) revealed the cholinergic component of contraction. Yohimbine (0.3 μM) increased the contraction almost to the same degree, an effect reversed by cyclopentolate (1 μM).
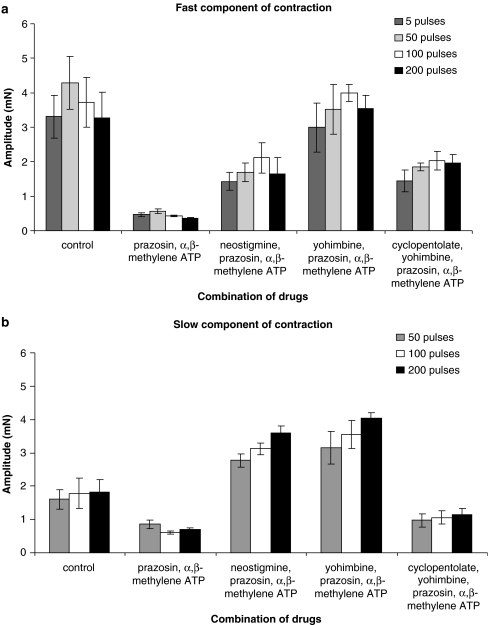
Figure 3.
Effects of different drugs on mouse vas deferens contraction. (a) Graphs summarising the control amplitude of the fast component of contraction in the mouse vas deferens, evoked by trains of 5, 50, 100 and 200 stimuli and in the presence of combinations of drugs: prazosin (0.1 μM), α,β-methylene ATP (1 μM), neostigmine (10 μM), yohimbine (0.3 μM) and cyclopentolate (1 μM). (b) Graphs summarising the control amplitude of the slow component of contraction in the mouse vas deferens, evoked by trains of 50, 100 and 200 stimuli and in the presence of combinations of drugs: prazosin (0.1 μM), α,β-methylene ATP (1 μM), neostigmine (10 μM), yohimbine (0.3 μM) and cyclopentolate (1 μM).
Effects of neostigmine
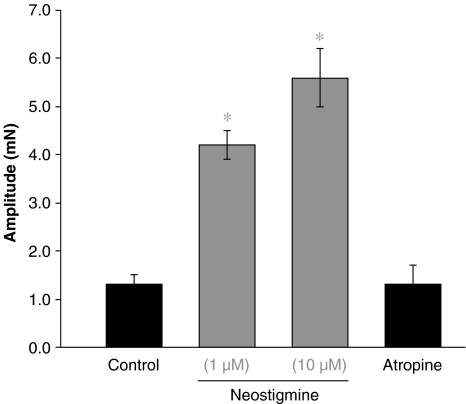
In controls, neostigmine (Figure 5; 1 and 10 μM, n=6, P<0.05) powerfully increased the amplitude of the second component of contraction (the ‘hump': initial mean amplitude, 50 stimuli: 1.4±0.2 mN; 100 stimuli: 1.7±0.4 mN; 200 pulses: 1.4±0.3 mN) in all experiments with trains of 50 stimuli (mean amplitude of the ‘hump' 3.6±0.5 mN, 1 μM and 3.7±0.7 mN, 10 μM), 100 stimuli (mean amplitude of the ‘hump' 4.2±0.5 mN, 1 μM and 4.6±0.5 mN, 10 μM) and 200 stimuli (mean amplitude of the ‘hump' 4.3±0.4 mN, 1 μM and 5.1±0.5 mN, 10 μM), the ‘twitch' being unaffected. Characteristically, the second component of contraction always exhibited rhythmic oscillations during the period of stimulation. The increase in amplitude of the ‘hump' was reversed to control levels by the addition of either cyclopentolate (Figure 4; 1 μM, n=6, P<0.05) or atropine (Figure 5; 1 μM, n=6, P<0.05).
Figure 5.
Effect of neostigmine on the slow component of the electrically evoked contraction. Histogram showing the effect of neostigmine on the slow component of the response to electrical stimulation (train of 200 stimuli at 10 Hz). Both concentrations of neostigmine (n=6) increased the slow component significantly (P<0.05), recorded 60 min after drug application. After wash out of neostigmine and subsequent application of atropine (1 μM; n=6), the contraction returned to control levels.
Figure 4.
Effect of neostigmine and cyclopentolate on the electrically evoked contraction. Typical traces showing contractions evoked by trains of 5 (a), 50 (b), 100 (c) and 200 stimuli (d) at 10 Hz. Stimuli of 50 pulses or more resulted in contractions comprised of two components. Neostigmine (1 μM) increased the slow component, an effect reversed by cyclopentolate (1 μM). Drugs were allowed to equilibrate for 60 min before measurements were made.
Effects of cyclopentolate and atropine
In the absence of bretylium, neither cyclopentolate (0.1 and 1 μM, n=6, P>0.05) nor atropine (1 μM, n=6, P>0.05) affected the neurogenic contraction, regardless of the number of stimuli in a train (5, 50, 100 or 200). However, neostigmine (10 μM, n=12, P<0.05), applied afterwards, increased the amplitude of the ‘hump' evoked by trains of 50, 100 and 200 stimuli in all experiments (mean amplitude 6.7±0.7, 6.6±0.3, 6.6±1.0 mN, respectively).
Effects of Ach
Ach (10 μM) applied alone (mean amplitude 3.1±0.3 mN, n=6) or in the presence of neostigmine (mean amplitude 5.0±0.9 mN, n=6) elicited a contraction in the aganglionic mouse vas deferens. Ach also enhanced the contraction evoked by 200 stimuli (Figure 6; mean amplitude of the ‘twitch' 9.0±1.2 mN and ‘hump' 8.5±0.6 mN, n=6, P>0.05 for Ach alone; in the presence of neostigmine (10 μM), mean amplitude of the ‘twitch' 10.8±1.4 mN and ‘hump' 12.6±0.7 mN, n=6, P<0.05). In the presence of cyclopentolate, Ach did not elicit contraction (mean amplitude reduced from 6.1±0.37 mN to 0, n=6).
Figure 6.
Effects of Ach and neostigmine on mouse vas deferens contraction. Traces showing contractions evoked by trains of 200 pulses (10 Hz). Neostigmine (10 μM) increased the electrically evoked contraction. Ach (10 μM), either given alone or in the presence of neostigmine, elicits a contraction in the mouse vas deferens. Note that Ach enhances the electrically evoked contractions.
Discussion
The common view that ATP and NA are the only important neurotransmitters controlling motility in the mouse vas deferens has to be reassessed. Neurogenic contraction of the longitudinal layer of the mouse vas deferens, elicited by long trains of stimuli, is usually thought of as being mediated solely by ATP and NA released from sympathetic nerves. Surprisingly, a TTX-sensitive residual component of evoked contraction was evident even after 5 h exposure to bretylium (Figure 1). It seems unlikely that this residual component reflected bretylium-resistant NA or ATP release, as the control ‘twitch' and ‘hump' responses were abolished within 2 h of bretylium application. On the other hand, the same component could be revealed in the presence of prazosin, an α1-adrenoceptor antagonist, and α,β-methylene ATP, a P2X1 receptor agonist, by both neostigmine and yohimbine, an α2-adrenoceptor antagonist. The component revealed either by neostigmine or yohimbine was blocked by cyclopentolate.
The recent characterisation (Majcen, 1984; Ventura et al., 1998) of a cholinergic innervation of the rat vas deferens prompted the idea that cholinergic transmission was responsible for the bretylium-resistant component of contraction. The observations that the abolition of the residual component by cyclopentolate – a competitive, mAchR antagonist – and the reversal of this inhibition by neostigmine – a cholinesterase inhibitor – together provide strong evidence to support a cholinergic basis of the residual component, mediated through mAchRs.
Organ bath studies have demonstrated that nicotine increased the residual contraction only when added after cyclopentolate and neostigmine and occasionally when given in the presence of both of these drugs. The potentiation of the bretylium-insensitive contractions by nicotine suggests that facilitatory nAchRs are present on cholinergic nerve terminals. Brain et al. (2001) have shown that nicotine induces spontaneous Ca2+ transients in all varicosities of the mouse vas deferens. Moreover, Jackson & Cunnane (2002) have described bretylium-resistant evoked Ca2+ transients in the mouse vas deferens. Surprisingly, in previous confocal studies nicotine was shown to induce asynchronous Ca2+ transients in both bretylium-sensitive and bretylium-resistant varicose nerve terminals (unpublished data). There are four possible explanations. First, the varicosities in which bretylium failed to affect Ca2+ transients may be sympathetic but located in regions in which the varicosities do not possess enough functional uptake-1 sites for bretylium to block action potential propagation. Second, bretylium may block sympathetic nerves by a mechanism that does not involve blockade of action potential conduction in nerve terminals. Third, the varicosities in which bretylium failed to affect the transients may be cholinergic, or fourth, they may be sensory nerves.
Fluorescent histochemical studies have revealed that 12–20% of terminals have agranular vesicles in the mouse vas deferens, a feature characteristic of cholinergic endings. Indeed some of the cholinergic-type terminals were found apposed to smooth muscles cells (Yamauchi & Burnstock, 1969).
Under physiological conditions, it appears that cholinergic transmission is inhibited by sympathetic nerves, as cyclopentolate and atropine do not affect the control neurogenic contraction even when contractions were evoked by 200 stimuli. Nevertheless, by blocking cholinesterase, the amount of residual Ach becomes sufficient to increase significantly the electrically evoked contraction, even when the effects of sympathetic nerves were not blocked. The need for long trains of stimuli may be due to the fact that cholinergic nerves represent only 12–20% of the vas deferens innervation compared with sympathetic nerves, so a sustained stimulation is required in order to be able to influence the neurogenic contraction. The latency of the onset of the residual contraction is most likely explained by a G-protein-coupled mechanism, the M3 receptor (mAchR) being the most likely candidate.
In rodent vas deferens, two subtypes of mAchRs have been described (Silva et al., 1988): postjunctional mAchRs M3, involved in the potentiation of the neurogenic contraction (Matsuno & Mita, 1992), prejunctional inhibitory mAchR M1 (Miranda & Wolstenholme, 1985; Miranda et al., 1987, 1994, 1995) and, in addition, facilitatory nAchRs (Todorov et al., 1991) on sympathetic nerve terminals. In the present study, the subtype of the mAchRs is unknown as we have not made any attempt to characterise them further. Thus, the mouse vas deferens seems to be innervated by functional cholinergic nerves, whose action is limited by cholinesterase and can be modulated by the activation of nAchRs or inhibition of mAchRs. A schematic model of the neurogenic contraction of the mouse vas deferens is shown in Figure 7.
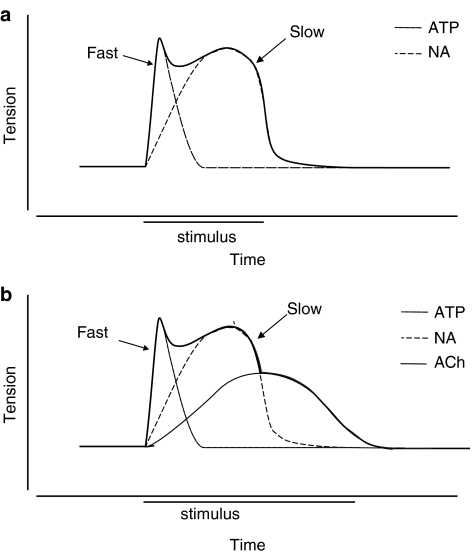
Figure 7.
(a) Schematic illustration showing the biphasic response to electrical stimulation in vas deferens. The ‘twitch' (fast) is selectively abolished by suramin, a P2X receptor antagonist, and therefore reflects the action of ATP. The ‘hump' (slow) is blocked by the α1-adrenoceptor antagonist prazosin and reflects the action of noradrenaline. Modified from Von Kugelgen & Starke (1991a, 1991b). (b) Diagram illustrating the third cholinergic component of contraction evoked by nerve stimulation.
Further investigations are required in order to determine for certain the exact locations of the nAchRs and mAchRs that affect the cholinergic transmission. It is known that the longitudinal layer of the mouse vas deferens is only a few smooth muscle cells thick and that the cholinergic innervation is believed to be mainly confined to the circular smooth muscle layer (Majcen, 1984; Ventura et al., 1998). The functional significance of the cholinergic innervation remains to be determined.
In conclusion, it has been demonstrated that there is a functional cholinergic innervation of the mouse vas deferens that is mediated through mAchRs, limited by cholinesterase action and can be modulated by activation of nAchRs.
Acknowledgments
A.M.C. was a Blaschko Fellow. We would also like to thank The Wellcome Trust for financial support.
Abbreviations
- Ach
acetylcholine
- ATP
adenosine triphosphate
- CGRP
calcitonin gene-related peptide
- mAchR
muscarinic receptor
- NA
noradrenaline
- nAchRs
nicotinic receptors
- TTX
tetrodotoxin
- VIP
vasointestinal peptide
References
- ABERDEEN J., CORR L., MILNER P., LINCOLN J., BURNSTOCK G. Marked increases in calcitonin gene-related peptide-containing nerves in the developing rat following long-term sympathectomy with guanethidine. Neuroscience. 1990;35:175–184. doi: 10.1016/0306-4522(90)90132-n. [DOI] [PubMed] [Google Scholar]
- BRAIN K.L., TROUT S.J., JACKSON V.M., DASS N., CUNNANE T.C. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]
- CARNEIRO R.C., MARKUS R.P., DUBOCOVICH M.L. Presynaptic modulation by melatonin of the nicotinic-induced calcium-dependent release of norepinephrine from the rat vas deferens. Biol. Signals. 1993;2:199–206. doi: 10.1159/000109493. [DOI] [PubMed] [Google Scholar]
- DIXON J.S., GOSLING J.A. The distribution of autonomic nerves in the musculature of the rat vas deferens. A light and electron microscope investigation. J. Comp. Neurol. 1972;147:175–188. doi: 10.1002/cne.901460204. [DOI] [PubMed] [Google Scholar]
- GEPPETTI P., FRILLI S., RENZI D., SANTICIOLI P., MAGGI C.A., THEODORSSON E., FRANCIULLACCI M. Distribution of calcitonin gene-related peptide-like immunoreactivity in various rat tissues: correlation with substance P and other tachykinins and sensitivity to capsaicin. Regul. Pept. 1988;23:289–298. doi: 10.1016/0167-0115(88)90229-7. [DOI] [PubMed] [Google Scholar]
- HÖKFELT T.G., LJUNGDAHL A.S. Histochemical determination of neurotransmitter distribution. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1972;50:1–24. [PubMed] [Google Scholar]
- JACKSON V.M., CUNNANE T.C. Bretylium or 6-OHDA-resistant, action potential-evoked Ca2+ transients in varicosities of the mouse vas deferens. Br. J. Pharmacol. 2002;135:1845–1850. doi: 10.1038/sj.bjp.0704677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUJAT R., ROSE C., ROOTS L. A ‘direct-coloring' thiocholine method for cholinesterases. J. Histochem. Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- MAJCEN Z. Cholinesterases and choline acetyltransferase in the ductus deferens of the guinea pig. Histochemistry. 1984;81:195–199. doi: 10.1007/BF00490117. [DOI] [PubMed] [Google Scholar]
- MARKUS R.P., ZAGO W.M., CARNEIRO R.C. Melatonin modulation of presynaptic nicotinic acetylcholine receptors in the rat vas deferens. J. Pharmacol. Exp. Ther. 1996;279:18–22. [PubMed] [Google Scholar]
- MATSUNO K., MITA S. Involvement of the muscarinic receptors in the postsynaptic potentiation of neurogenic twitch contraction in the mouse vas deferens. Life Sci. 1992;50:799–806. doi: 10.1016/0024-3205(92)90185-r. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., DURAN E., BUSTAMANTE D., PAEILE C., PINARDI G. Pre- and postjunctional muscarinic receptors subtypes in the vas deferens of rat. Gen. Pharmacol. 1994;25:1643–1647. doi: 10.1016/0306-3623(94)90366-2. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., DURAN E., FERNANDEZ E., PINARDI G. Muscarinic receptors subtypes in the bisected vas deferens of the rat. Gen. Pharmacol. 1995;26:387–391. doi: 10.1016/0306-3623(94)00185-p. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., WOLSTENHOLME W.W. Presynaptic regulation of Ach of the NE mediated responses in the rat vas deferens. J. Recept. Res. 1985;5:231–243. doi: 10.3109/10799898509041881. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., WOLSTENHOLME W.W., MOREU G.M., SANTIAGO P.A. Ontogenesis of autonomic receptors and AchE activity in the rat vas deferens. Gen. Pharmacol. 1987;18:425–429. doi: 10.1016/0306-3623(87)90102-9. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., WOLSTENHOLME W.W., MOREU G.M., SANTIAGO P.A. Effects of haloperidol on neurotransmitter activity in the rat vas deferens. Gen. Pharmacol. 1988;19:123–127. doi: 10.1016/0306-3623(88)90017-1. [DOI] [PubMed] [Google Scholar]
- MORRIS J.L., GIBBINS I.L. Co-Transmission and Neuromodulation, in Autonomic Neuroeffector Mechanisms. Chur, Switzerland: Harwood Academic Publishers; 1992. pp. 33–121. [Google Scholar]
- SAITO A., TOMOBE Y., GOTO K. Effect of capsaicin on smooth muscles of rat vas deferens: involvement of calcitonin gene-related peptide. J. Pharmacol. Exp. Ther. 1987;242:665–672. [PubMed] [Google Scholar]
- SILVA W.I., WOLSTENHOLME W.W., MIRANDA H.I. Pre-and postsynaptic muscarinic receptors of the rat vas deferens: an update. P. R. Health Sci. J. 1988;7:105–110. [PubMed] [Google Scholar]
- STARKE K., BULTMANN R., BULLOCH J.M., VON KULGELGEN I. Noradrenaline-ATP corelease and cotransmission following activation of nicotine receptors at postganglionic sympathetic axons. J. Neural. Trans. Suppl. 1991;34:93–98. doi: 10.1007/978-3-7091-9175-0_12. [DOI] [PubMed] [Google Scholar]
- TODOROV L., WINDISCH K., SHERSEN H., LAJITHA A., PAPASOVA M., VIZI E.S. Prejunctional nicotinic receptors involved in facilitation of stimulation-evoked noradrenaline release from the vas deferens of the guinea pig. Br. J. Pharmacol. 1991;102:186–190. doi: 10.1111/j.1476-5381.1991.tb12151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTURA S., BAVETTA S., MILNER P., RALEVIC V., BURNSTOCK G. Nitric oxide synthase is co-localized with vasoactive intestinal polypeptide in postganglionic parasympathetic nerves innervating the rat vas deferens. Neuroscience. 1998;83:607–616. doi: 10.1016/s0306-4522(97)00416-8. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., STARKE K. Release of noradrenaline and ATP by electrical stimulation and nicotine in guinea pig vas deferens. Naunyn-Schmiedebergs Arch. Pharmacol. 1991a;344:419–429. doi: 10.1007/BF00172581. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., STARKE K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol. Sci. 1991b;12:319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- WANIGASEKARA Y., KEPPER M.E., KEAST J.R. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311:175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- YAMAUCHI A., BURNSTOCK G. Post-natal development of the innervation of the mouse vas deferens. A fine structural study. J. Anat. 1969;104:17–32. [PMC free article] [PubMed] [Google Scholar]
- ZAGO W.M., MARKUS R.P. Melatonin modulation of presynaptic nicotinic acetylcholine receptors located on short noradrenergic neurons of the rat vas deferens: a pharmacological characterization. Braz. J. Med. Biol. Res. 1999;32:999–1006. doi: 10.1590/s0100-879x1999000800010. [DOI] [PubMed] [Google Scholar]