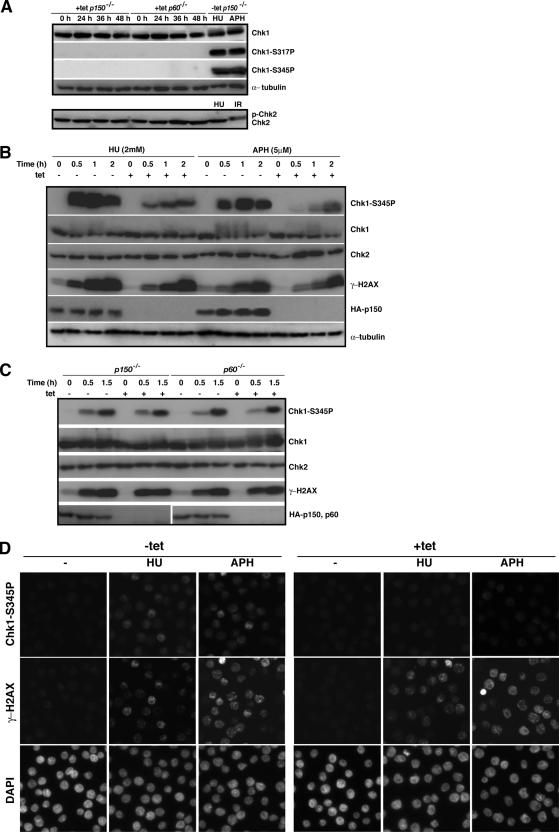
Figure 4.
Checkpoint activation in p150- and p60-deficient cells. (A) The p150- and p60-conditional knockout cells were cultured in the presence (+tet) of tet for indicated times, and whole cell extracts were prepared for Western blotting, by using antibodies for Chk1, phosphorylated Chk1 at Ser317 (Chk1-S317P), phosphorylated Chk1 at Ser345 (Chk1-S345P), Chk2, and α-tubulin as a loading control. Extracts from the p150-conditional knockout cells exposed to 2 mM HU and 5 μM APH for 1 h, or x-ray irradiation (IR; 5 Gy) in the absence of tet (−tet) were also subjected to Western blotting to examine whether Chk1 and Chk2 activations are intact in the p150-conditional knockout cells. (B) The p150-conditional knockout cells were cultured in the presence (+) or absence (−) of tet for 24 h, and treated with 2 mM HU or 5 μM APH for indicated times. The same amounts of whole cell extracts were analyzed by Western blotting, by using antibodies for phosphorylated Chk1 at Ser345 (Chk1-S345P), Chk1, Chk2, γ-H2AX, HA, and α-tubulin as a loading control. Antibody for HA was used to confirm the p150 depletion. (C) The p150- and p60-conditional knockout cells were cultured in the presence (+) or absence (−) of tet for 24 h and irradiated with UV light (UV-C; 3 J/mm2) for indicated times. Western blotting was performed as described in A. Antibody for HA was used to confirm the depletion of p150 and p60. (D) The p150-conditional knockout cells were cultured in the presence (+tet) or absence (−tet) of tet for 24 h, treated with 1 mM HU or 5 μM APH 1.5 h and examined by immunofluorescence microscopy by using antibodies for phosphorylated-Chk1 at Ser345 (Chk1-S345P) (top) and γ-H2AX (middle). Nuclei were counterstained with DAPI (bottom). More than 300 cells were examined at each time to determine the percentage of cells displaying signals of phosphorylated Chk1 and γ-H2AX.