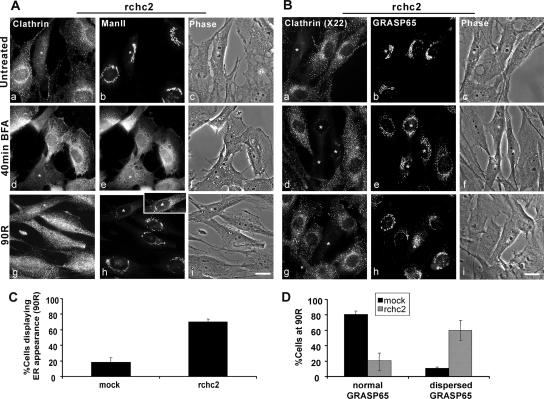
Figure 6.
Clathrin heavy chain knockdown prevents Golgi reassembly after BFA treatment. NRK cells transfected with rchc2 siRNA for 3.5 d were untreated or treated with 5 μg/ml BFA for 40 min (40 min BFA), followed by 90 min washout (90R) after which Golgi morphology was determined using either a mouse monoclonal anti-ManII and rabbit anti-clathrin antibody (A) or a rabbit anti-GRASP65 antibody in conjunction with the monoclonal anti-CHC, X22 antibody (B). BFA-treated cells: (A) ManII redistributes into the ER in rchc2-transfected or untransfected cells (40 min BFA; e) but fails to regain its juxtanuclear localization in rchc2 containing cells (90R; h); inset, because of its lower fluorescence intensity than the surrounding cells, the levels of the CHC knockdown cell were adjusted to reveal ER-like staining. (B) GRASP65 redistributes independently of the ER and maintains a loose juxtanuclear localization in rchc2-transfected and untransfected cells (40 min BFA; e); however, it does not regain its lace-like structure (90R; h). Asterisks indicate siRNA-transfected cells. Bar, 20 μm. (C) Golgi morphologies were quantified by counting cells that displayed either Golgi or ER-like localization of ManII in nonsilencing siRNA or rchc2-treated samples at 90-min washout (90R). The results were expressed as the average percentage of cells displaying ER distribution. (D) GRASP65-positive Golgi morphologies were defined as normal and dispersed and quantified as in C. The frequency of each morphology in mock- and rchc2-transfected cells at 90-min washout (90R) is expressed as the percentage of cells displaying each phenotype. Cells were scored by two independent observers from three experiments.