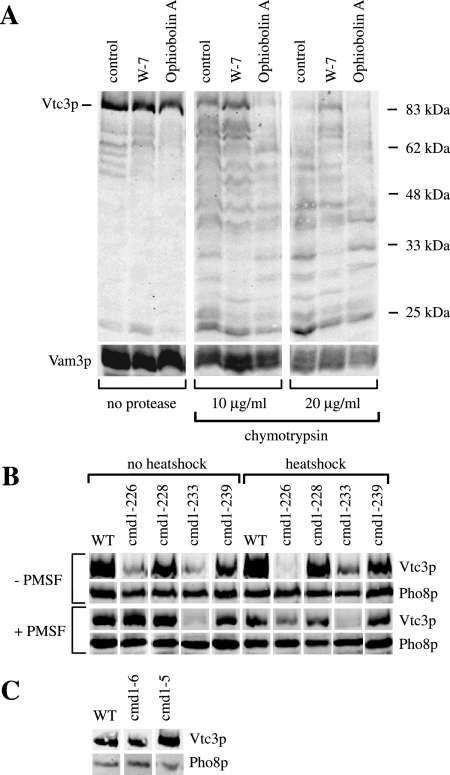
Figure 6.
(A) Limited proteolysis of Vtc3p. In vitro microautophagic reactions were run without any inhibitor (control) or with 400 μM W-7 or 100 μM ophiobolin A. After 1 h at 27°C, the reactions were chilled on ice and stopped by dilution with 600 μl 150 mM KCl in PS buffer. Vacuoles were sedimented (6500 × g, 6 min, 2°C) and resuspended in 500 μl 150 mM KCl in PS buffer. Chymotrypsin was added to final concentrations as indicated and incubated for 10 min on ice. Digestion was stopped by chloroforme-methanol precipitation. Proteins were analyzed by SDS-PAGE and Western blotting against Vtc3p and the vacuolar SNARE protein Vam3p (control). (B) Temperature-sensitive mutants. Yeast cells were grown in log phase at 25°C in YPD. For heat-shock treatment part of the cultures were transferred to 37°C for 1 h. Cells were harvested and spheroplasted at 30°C (no heat shock) or at 37°C (heat shock) in presence or absence of 1 mM PMSF, and vacuoles were prepared. Vacuoles (50 μg protein) were sedimented (20,000 × g, 5 min, 2°C), resuspended in 25 μl of sample buffer, heated to 95°C for 10 min, and analyzed by SDS-PAGE on a 10% gel and Western blotting against the N-terminal SPX-domain of Vtc3p or against the lumenal vacuolar protein Pho8p (alkaline phosphatase) as a loading control. (C) Mutants in the Ca2+-binding sites. Yeast cells were grown in log phase at 30°C in YPD, and vacuoles were prepared at 30°C. Vacuoles (30 μg of protein) were sedimented (20,000 × g, 5 min, 2°C), resuspended in 25 μl of sample buffer, heated to 95°C for 10 min, and analyzed as in B.