Abstract
The effect of ACh on the release of adenosine was studied in rat whole carotid bodies, and the nicotinic ACh receptors involved in the stimulation of this release were characterized.
ACh and nicotinic ACh receptor agonists, cytisine, DMPP and nicotine, caused a concentration-dependent increase in adenosine production during normoxia, with nicotine being more potent and efficient in stimulating adenosine release from rat CB than cytisine and DMPP.
D-Tubocurarine, mecamylamine, DHβE and α-bungarotoxin, nicotinic ACh receptor antagonists, caused a concentration-dependent reduction in the release of adenosine evoked by hypoxia. The rank order of potency for nicotinic ACh receptor antagonists that inhibit adenosine release was DHβE>mecamylamine>D-tubocurarine>α-bungarotoxin.
The effect of the endogenous agonist, ACh, which was mimicked by nicotine, was antagonized by DHβE, a selective nicotinic receptor antagonist.
The ecto-5′-nucleotidase inhibitor AOPCP produces a 72% inhibition in the release of adenosine from CB evoked by nicotine.
Taken together, these data indicate that ACh induced the production of adenosine, mainly from extracellular ATP catabolism at the CB through a mechanism that involves the activation of nicotinic receptors with α4 and β2 receptor subunits.
Keywords: Adenosine, acetylcholine, nicotinic acetylcholine receptors, ATP, carotid body, chemoreceptors
Introduction
Carotid bodies (CB) are major peripheral chemoreceptor organs that release neurotransmitters in response to hypoxia, generating action potentials at the carotid sinus nerve (CSN), which are integrated in the brainstem to induce a hyperventilatory compensatory response. CB glomus cells (putative chemosensory cells) contain several neurotransmitters, mainly catecholamines (for a review see Gonzalez et al., 1994), but the excitatory effects on CB chemotransduction have been attributed to adenosine (McQueen & Ribeiro, 1981; Monteiro & Ribeiro, 1987), ATP (Zhang et al., 2000; Rong et al., 2003) and acetylcholine (ACh) (Fitzgerald, 2000).
ACh nicotinic receptors are present at the CB in glomus cells (Dinger et al., 1981; Dasso et al., 1997; Obeso et al., 1997) and in nerve fibres (Shirahata et al., 1998). Immunohistochemical and RT–PCR techniques in the cat have shown the presence of α3, α4 and β2 subunits in glomus cells (for a review see Higashi et al., 2003) and α7 subunits in nerve fibres surrounding the glomus cells (Shirahata et al., 1998). In mice, transcripts of six nicotinic ACh receptor subunits, α3, α4, α5, α7, β2 and β4, were detected in CB total RNA (Cohen et al., 2002). The physiological function of these receptors is not completely understood, but they may be involved in the hyperpnoea caused by exogenous nicotine (Nic) (Fernández et al., 2002). Until the 1990s, nicotinic receptors were not considered fundamental for the O2 sensing mechanism at the CB because classical nicotinic blockers suppressed the excitatory actions of nicotinic agonists, but only reduced in a variable percentage the activation produced by natural stimuli (McQueen 1977; 1983). More recently, Nurse and co-workers (Zhang et al., 2000) showed that the application of nicotinic ACh antagonists, like mecamylamine or hexamethonium, in co-cultures of glomus cells and ‘juxtaposed' petrosal ganglions only partially inhibits the hypoxia-evoked excitatory postsynaptic responses. Nevertheless, it is agreed that nicotinic ACh receptors in glomus cells act as modulators, increasing [Ca2+]i (Dasso et al., 1997) and inducing the release of neurotransmitters at the CB, such as dopamine (Obeso et al., 1997).
ATP and adenosine are released by the CB of the rat in response to hypoxia (Buttigieg & Nurse, 2004; Conde & Monteiro, 2004) and have been proposed as excitatory neurotransmitters at the CB in animal models. The involvement of adenosine in CB chemotransduction has been also shown in humans. Intravenous administration of adenosine in man causes hyperventilation, dyspnoea and chest discomfort attributed to activation of CB chemoreceptors (Watt & Routledge, 1985; Watt et al., 1987; Uematsu et al., 2000) and both adenosine and its antagonists modify the hyperventilatory responses to hypoxia but not to hypercapnia (Maxwell et al., 1986; 1987). The importance of adenosine in CB chemotransduction would be reinforced if it were also involved in the chemotransduction mechanism initiated by substances like Nic/ACh that apparently mimic the excitatory effect of hypoxia on CSN activity and/or ventilation.
In the present work, it was postulated that the excitatory effects of ACh at the CB could involve the release of other excitatory neurotransmitters and the hypothesis that activation of nicotinic ACh receptors can stimulate adenosine release at the CB was tested. To test this hypothesis, whole CBs from rats were used and a pharmacological functional characterization of the nicotinic receptors was performed. Since adenosine at the CB can originate from both release through nucleoside transporters and extracellular catabolism of ATP (Conde & Monteiro, 2004), the contribution of extracellular ATP degradation to adenosine production induced by nicotinic stimulation was also studied.
Brief accounts of some of the results in this study have been published previously (Conde & Monteiro, 2003).
Methods
Animals and surgical procedures
Experiments were performed in Wistar adult rats (250–350 g) from the Faculty of Medical Sciences animal house, kept at a constant temperature (21°C) and with a regular light (0800–2000 h) and dark (2000–0800 h) cycle, with food and water ad libitum. Rats were anaesthetized with sodium pentobarbital (60 mg kg−1 intraperitoneal (i.p.)), underwent tracheotomy and were breathing unassisted during the surgical procedure (duration approximately 15 min). Carotid bodies were removed in situ under a Nikon SMZ-2B dissection scope and placed in ice-cold Tyrode solution (in mM: NaCl 116; NaHCO3 24; KCl 5; CaCl2 2; MgCl2 1.1; HEPES 10; glucose 5.5) adjusted to pH 7.40 and equilibrated with 95% O2+5% CO2. After removal of the carotid bodies, the rats were killed by an intracardiac injection of a lethal dose of pentobarbital. Animal handling and experiments complied with the European Union directives (Portuguese law nos. 1005/92 and 1131/97). After 30 min in hyperoxia (95% O2+5% CO2) (recovery period) at 37°C, the CBs were incubated for 10 min in Tyrode solution equilibrated with 20% O2+5% CO2 (normoxia) or 10% O2+5% CO2 (hypoxia) and in the presence of 2.5 μM of erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), an inhibitor of adenosine deaminase, to avoid adenosine degradation.
Effect of ACh nicotinic receptor agonists on adenosine released from CBs
The effect of ACh nicotinic receptor agonists was assessed in normoxic conditions (20% O2+5% CO2). Nicotinic receptor agonists used were ACh (30 μM), cytisine (Cyt) (0.01–10 μM), dimethylphenylpiperazinium (DMPP) (0.1–300 μM) and Nic (0.1–100 nM). In each experiment, only one agonist concentration was tested and added to the incubation medium that contained 2.5 μM of EHNA, in the last 3 min of the incubation period (10 min) to avoid nicotinic ACh receptor desensitization. In the experiments with ACh, an inhibitor of AChE, physostigmine was added to the incubation medium and two different concentrations (30 and 300 μM) were tested.
After the incubation period, the CBs were removed from the medium and the nucleotides were extracted from the incubation medium.
Effect of ACh nicotinic receptor antagonists on adenosine released from CBs
The effect of ACh nicotinic receptor antagonists was assessed in hypoxic conditions (10% O2+5% CO2). Endogenous ACh released by the CB in response to its physiological stimulus, hypoxia, may activate nicotinic ACh receptors different from those stimulated by exogenously applied agonist. However, only the characterization of nicotinic ACh receptors that are involved in the release of adenosine under physiological conditions was considered here. The dose–response curves for the effects of D-tubocurarine (0.001–200 μM), mecamylamine (0.001–100 μM), dihydro-β-erythroidine (DHβE, 0.001–100 μM) and α-bungarotoxin (0.001–10 μM) on the release of adenosine were performed in CBs stimulated by 10 min of hypoxia. The ACh nicotinic receptor antagonists were included in the medium at the beginning of the recovery period in hyperoxia, in order to try to obtain an adequate diffusion of the drug and the block of the nicotinic receptors. After the incubation period in hypoxia, the CBs were removed and the nucleotides were extracted from the incubation medium.
Pharmacological demonstration of the involvement of neuronal nicotinic ACh receptors
To demonstrate the involvement of neuronal nicotinic receptors, the effect of the selective nicotinic receptor antagonist, DHβE, on the release of adenosine from CBs was assessed by incubating CBs with a maximal dose (100 μM) of DHβE in normoxia (20% O2+5% CO2) for 10 min in the presence of Nic (0.1–1 μM). After the incubation period, the CBs were removed and the nucleotides were extracted from the incubation medium.
Effect of extracellular ATP catabolism inhibitor on the release of adenosine evoked by Nic
The effect of the inhibitor of ecto-5′-nucleotidase, α,β-methylene ADP (AOPCP) on the release of adenosine from CBs was assessed by incubating CBs with 100 μM of AOPCP in normoxia (20% O2+5% CO2) for 10 min in the presence of 100 nM of Nic. After the incubation period, the CBs were removed and the nucleotides were extracted from the incubation medium.
Nucleotide extraction and HPLC analysis
Nucleotides were extracted from the medium following a protocol described by Cunha et al. (1994). Aliquots of neutralized supernatant were collected and kept at −20°C until analysis by HPLC. The samples were analysed in triplicate by reverse-phase HPLC with UV detection at 254 nm as described previously (Conde & Monteiro, 2004).
Drugs and chemicals
ACh, adenosine, AOPCP, α-bungarotoxin, Cyt, DHβE, DMPP, EHNA, mecamylamine, Nic, D-tubocurarine, physostigmine and Sigmacote were all from Sigma (Portugal/Spain). Cytisine and physostigmine were made up in a 5 and 30 mM stock solution in DMSO and ethanol, respectively. All stock solutions were stored as frozen aliquots at −20°C. Dilutions of stock solutions were made in Tyrode solution in accordance with the drug concentration used.
Data analysis
The amount of adenosine present in the incubation medium was expressed in pmol CB−1. Data were evaluated using Graph Pad Prism Software, version 4 and were presented as mean±s.e.m. The significance of the differences between the means was calculated by unpaired Student's t-test. P-values of 0.05 or less were considered to represent significant differences.
Results
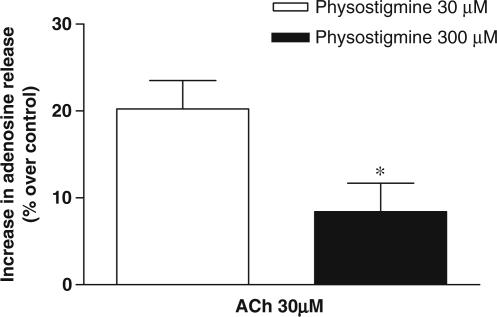
The basal amounts of adenosine released from rat CBs during 10 min of normoxia were 113.3±11.77 pmol CB−1 (n=5). The effect of ACh on the amount of adenosine released from intact rat CB in normoxia is shown in Figure 1. ACh (30 μM) plus physostigmine (300 μM) during 10 min caused a slight but not statistically significant increase to 122.75±15.33 pmol CB−1 (n=5), which corresponds to an increase of 8.34% in the amount of adenosine (Figure 1). Since it has been previously shown that in high concentrations (>100 μM) this AChE-inhibiting drug, physostigmine, is a competitive ligand of ACh on α4-containing nicotinic ACh receptors (Zwart et al., 2000), a lower concentration (30 μM) was tested. As can be observed in Figure 1, ACh (30 μM) induced a statistically significant increase of 20.2±3.3% (n=5) in the amount of adenosine released in the presence of 30 μM of physostigmine. To avoid the interaction of AChE inhibitors with nicotinic receptors, more stable agonists of nicotinic ACh receptors, namely Cyt, DMPP and Nic were tested.
Figure 1.
Effect of ACh (30 μM) on the amount of adenosine released from rat CBs in the presence of distinct concentrations of physostigmine (30 and 300 μM, n=5). All the experiments were performed in normoxia and in the presence of EHNA (2.5 μM). Zero per cent increases correspond to 113.30±11.77 pmol of adenosine/CB (n=5) in normoxia.*P<0.05; unpaired Student's t-test corresponding to the differences between the effects of distinct doses of physostigmine. Vertical bars represent means±s.e.m.
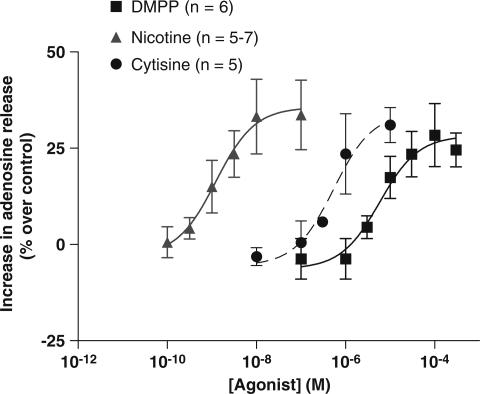
Cytisine (0.01–10 μM), DMPP (0.1–300 μM) and nicotine (0.1–100 nM) increased the release of adenosine in the CBs in normoxia in a concentration-dependent manner (Figure 2). Maximal increases (Emax) in the release of adenosine occurred with concentrations of 10 μM Cyt, 100 μM DMPP and 100 nM Nic. The EC50 (drug concentration that produced 50% of maximal effect) and Emax, obtained from dose–response curves of nicotinic agonists, are represented in Table 1.
Figure 2.
Dose–response curves for the effects of nicotinic ACh receptor agonists, Cyt (n=5), DMPP (n=6) and Nic (n=5–7) on adenosine released from rat CBs in normoxia. Zero per cent increases correspond to 113.3±11.77 pmol of adenosine/CB (n=5) in normoxia. Vertical bars represent means±s.e.m.
Table 1.
Efficacy and potency of nicotinic ACh receptor agonists in stimulating adenosine release at the CB
| Agonist | Emax (% effect) | EC50 (μM) |
|---|---|---|
| Cyt (n=5) | 34.15±6.8 | 0.54 |
| DMPP (n=6) | 28.27±4.1 | 5.8 |
| Nic (n=5/7) | 35.63±6.0 | 1.2 |
Cyt=cytisine; DMPP=dimethylphenylpiperazinium; Emax=maximal increase (%) in the release of adenosine (mean±s.e.m.); EC50=drug concentration that produced 50% of maximal effect; Nic=nicotine.
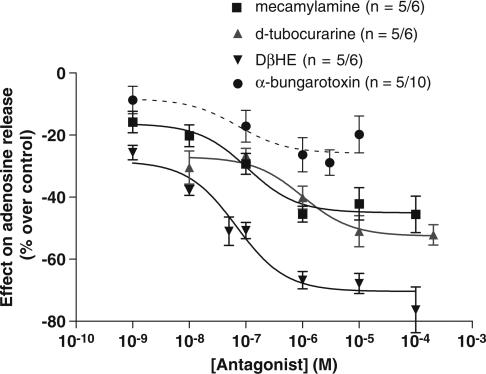
The effect of nicotinic ACh antagonists on the amount of adenosine released from rat CBs was assessed during physiological stimulation of chemoreceptor cells with hypoxia (10% O2). Dose–response curves for the effect of three nicotinic ACh receptor antagonists, D-tubocurarine, DHβE and α-bungarotoxin, as well for the effect of the allosteric inhibitor of nicotinic ACh receptor, mecamylamine, on adenosine release from rat CBs elicited by hypoxia are shown in Figure 3. As had been shown previously (Conde & Monteiro, 2004), hypoxia by itself increased the release of adenosine in the CB to 152.1±5.2 pmol CB−1 (0% effect, n=5). D-Tubocurarine (0.001–100 μM), mecamylamine (0.001–100 μM), DHβE (0.001–100 μM) and α-bungarotoxin (0.001–10 μM) caused a concentration-dependent decrease in the amount of adenosine released from the rat CBs in response to hypoxia, with the effect of α-bungarotoxin being very small (Emax=22.2±3.4%; Figure 3). The maximal inhibitory effect (Emax) on adenosine release was obtained with 100 μM of DHβE (Emax=70.39±2.4%; Figure 3). IC50 (drug concentrations that caused 50% of maximal effect) and Emax values obtained from the dose–response curves for the nicotinic ACh receptor antagonists are represented in Table 2. These results showed a rank order of potency with DHβE>mecamylamine>D-tubocurarine>α-bungarotoxin.
Figure 3.
Effects of nicotinic ACh receptor antagonists, α-bungarotoxin, D-tubocurarine and DHβE and of the allosteric inhibitor, mecamylamine, on the release of adenosine from rat CBs stimulated by hypoxia (10% O2). Zero per cent effect corresponds to 152.1±5.2 pmol of adenosine/CB (n=5) in response to hypoxia. Data are means±s.e.m. (n=5–6).
Table 2.
Efficacy and potency of nicotinic ACh antagonists in inhibiting the release of adenosine in CBs stimulated by hypoxia
| Antagonist | Emax (% effect) | IC50 (μM) |
|---|---|---|
| α-Bungarotoxin | −22.2±3.4 | — |
| D-Tubocurarine | −52.56±2.7 | 1.06 |
| Mecamylamine | −45.03±2.8 | 0.10 |
| DHβE | −70.39±2.4 | 0.067 |
DHβE=dihydro-β-erythroidine; Emax=maximal % of inhibition (mean±s.e.m.); IC50=drug concentrations that caused 50% of the maximal inhibition.
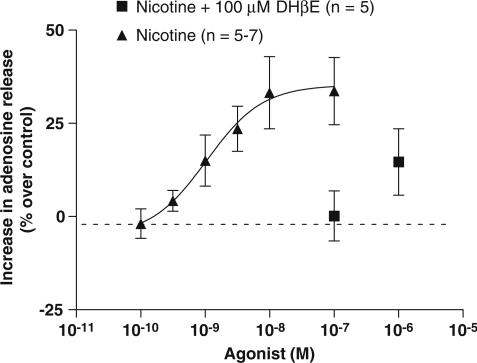
To demonstrate the involvement of neuronal nicotinic receptors, the effect of the selective nicotinic receptor antagonist, DHβE, on the release of adenosine from CBs evoked by Nic was studied. It was observed that DHβE inhibits the effect of Nic on adenosine release from CB, and this inhibition was complete when the concentration of Nic (100 nM) that produces the maximal effect on adenosine release from CB was used. To observe increases in adenosine release from CB evoked by Nic in the presence DHβE, it was necessary to increase the dose of Nic applied (Figure 4). Looking at Figure 4, it seems that the inhibition by DHβE moves the dose–response curve for the release of adenosine evoked by Nic to the right.
Figure 4.
Effect of the selective nicotinic receptor antagonist, DHβE (100 μM) on the release of adenosine evoked by Nic (0.1–1 μM) during normoxia (20% O2+5% CO2). Values represent means±s.e.m. (n=5).
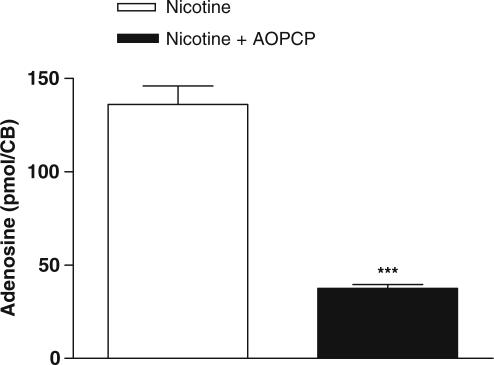
In order to investigate whether nicotinic ACh receptor activation induced the release of adenosine per se or induced the release of ATP (this being the nucleotide that is further metabolized into adenosine), experiments in CBs stimulated by Nic in a concentration (100 nM) that induced a maximal release of adenosine were performed in the presence of the inhibitor of ecto-5′-nucleotidase, AOPCP. AOPCP (100 μM), used in a concentration 10 times higher than that which inhibits 90% of AMP hydrolysis by ecto-5′-nucleotidases (Meghji & Burnstock, 1995), reduced the extracellular accumulation of adenosine by 72% (P<0.001) in the CBs stimulated by Nic (Figure 5).
Figure 5.
Effect of 100 μM of AOPCP on the release of adenosine in CBs stimulated by 100 nM of Nic in normoxia. ***P<0.001; unpaired Student's t-test corresponding to the differences observed in the presence or absence of AOPCP. Data are means±s.e.m. (n=5).
Discussion
Activation of ACh nicotinic receptors at the CB in normoxia stimulated the release of adenosine that apparently comes mainly from extracellular degradation of ATP. The increase in the amount of adenosine at the CB induced by hypoxia was partially antagonized by ACh nicotinic receptor antagonists. The rank order of potency for the effect of nicotinic agonists and antagonists on the release of adenosine was Nic>Cyt>DMPP and DHβE>mecamylamine>D-tubocurarine>α-bungarotoxin.
The characterization of nicotinic receptor subunits in the CB of the rat has never been performed. However, in the cat, immunohistochemical and RT–PCR techniques have reported the presence of α3, α4 and β2 subunits in glomus cells (for a review see Higashi et al., 2003) and α7 subunits in nerve fibres surrounding the glomus cells (Shirahata et al., 1998). In this work, we have described the involvement of neuronal Nic ACh receptors in stimulating the release of adenosine from CBs and characterized the nicotinic ACh receptor involved. The effect of the endogenous agonist, ACh, was mimicked by Nic (nicotinic ACh receptor agonist), used in a concentration that induces a maximal release of adenosine, this effect being antagonized by DHβE (a selective nicotinic ACh receptor antagonist), demonstrating the involvement of a neuronal nicotinic ACh receptor in the induction of adenosine release from rat CBs. The rank order of potency obtained for the nicotinic ACh receptor agonists studied is compatible with nicotinic receptors that contain subunits α4β2 (Nic>Cyt>DMPP) (Alexander et al., 2004). The presence of nicotinic receptors containing α2 and α3 subunits can be excluded, since receptors containing α2 subunits have a comparable affinity for Cyt and Nic and lower affinity for DMPP and receptors containing α3 subunits have a higher affinity for DMPP than for Cyt (Alexander et al., 2004; Jensen et al., 2005). Concerning the effect of the nicotinic receptor antagonists, a rank order of potency of DHβE>D-tubocurarine>α-bungarotoxin was observed for the inhibition of the release of adenosine from CB during hypoxia. Mecamylamine was excluded from this pattern since it is a nonselective allosteric inhibitor of nicotinic receptors with major affinity for nicotinic receptors containing α3 subunits (Jensen et al., 2005). As was found for the agonists, the pattern of inhibition is compatible with the presence of nicotinic receptors with α4β2 subunits (Jensen et al., 2005). The absence of consistent effects of α-bungarotoxin inhibiting the release of adenosine from CB during hypoxia excludes the presence of nicotinic receptors with α7, α8, α9 and α10 subunits. These findings are in accordance with the description of both α4 and β2 subunit transcription in total CB RNA in mice (Cohen et al., 2002), in glomus cells (Higashi et al., 2003) and with nicotinic ACh receptor α4β2 subunits modulating the release of neurotransmitters in the human CNS (Champtiaux et al., 2003).
The experiments herein described were performed in whole CBs and do not provide evidence concerning the cell origin of adenosine and/or nicotinic receptor localization, but previous evidence suggests that they are present in glomus cells. Nicotinic ACh receptors with α4 and β2 subunits were described in glomus cells (Higashi et al., 2003) and could act as modulators, increasing [Ca2+]i (Dasso et al., 1997) and inducing the release of several neurotransmitters at the CB, like dopamine (Obeso et al., 1997) and ATP. Adenosine can be produced by different cells at the CB in response to nicotinic activation. However, in response to acute moderate hypoxia (10% O2) significant increases in the amount of adenosine were found at the CB but not in other structures – arterial tissue or superior cervical ganglions – present at the CB and devoid of chemosensitive properties (Conde & Monteiro, 2004), suggesting that adenosine originates from glomus-chemosensitive cells.
The maximal increase in the release of adenosine (36%) induced by nicotinic agonists was similar to that (35%) caused by acute moderate hypoxia in the same conditions, but nicotinic antagonists do not completely abolish the stimulatory effect of hypoxia on adenosine production. This evidence, together with the differences between the Emax found for D-tubocurarine and for DHβE, could indicate that other, different nicotinic receptor subunits could be involved. For example, there might be α4 and β2 subunits associated with α5 subunits, as is described in CNS in cells that release dopamine (Champtiaux et al., 2003). Another interpretation is that hypoxia can trigger adenosine production by two different mechanisms: one independent and another dependent on nicotinic receptor activation. Further investigations and more specific drug tools are needed to clarify this point, but promising data can be advanced based on the amount of adenosine obtained during hypoxia and nicotinic activation when extracellular catabolism of ATP was inhibited with AOPCP. Inhibition of ecto-5′-nucleotidases with AOPCP reduced (present work) the increase in extracellular adenosine accumulation induced by Nic by 72%, but in the same experimental conditions caused a reduction of only 44% in adenosine extracellular accumulation in response to hypoxia (Conde & Monteiro, 2004). Adenosine is a common pathway for the CB responses to both hypoxia and nicotinic activation, but while moderate hypoxia stimulates both intracellular production of adenosine and the release of ATP (Conde & Monteiro, 2004), nicotinic activation preferentially induced the release of ATP. The proportion of ATP molecules that can stimulate P2 receptors before being deactivated by ectonucleotidases is not known, but the classical studies performed by McQueen & Ribeiro (1983; 1986) strongly support the theory that part of the excitatory effects of ATP on CSN is mediated by its metabolite adenosine.
The reduction in adenosine release induced by ACh that is caused by high concentrations of physostigmine, and previous indications that physostigmine is a competitive ligand of ACh on nicotinic receptors that contain α4 subunits (Zwart et al., 2000), together support the characterization of the α4β2 nicotinic receptor as containing subunits that stimulate adenosine release at the CB.
Interactions between adenosine and ACh in the central nervous system and peripheral nerve endings have been described (for a review, see, e.g., Ribeiro et al., 1996). It is generally accepted that adenosine can act as a modulator of ACh release: selective activation of A1 and A2 adenosine receptor subtypes causes, respectively, inhibitory and excitatory effects on ACh release (Ribeiro et al., 1996). In whole cat carotid bodies, it was recently shown that exogenous adenosine stimulates the release of ACh in hypoxic (4% O2) or hyperoxic (40% O2) conditions (Fitzgerald et al., 2004). This work does not provide evidence on the type of adenosine receptors, mechanisms or cell types involved (Fitzgerald et al., 2004), but is consistent with the hypothesis that the effects of both excitatory transmitters – adenosine/ATP and ACh – act by synergistic mechanisms at the CB. The characterization of A2 receptors at the CB, localized in several structures (glomus cells, vessels, nerve endings) was extensively carried out in vitro (Monteiro et al., 1996; Gauda et al., 2000; Kobayashi et al., 2000) and in vivo (McQueen & Ribeiro, 1986; Ribeiro & Monteiro, 1991). It was recently demonstrated that at the CB ACh apparently comes from nerve endings instead of glomus cells (Gauda et al., 2004), which is compatible with its action on nicotinic receptors with α4 subunits in glomus cells, stimulating the release of ATP/adenosine. In contrast to the well-known modulatory role for adenosine in cholinergic transmission, we are not aware of information relating to the consequences of nicotinic receptor activation on adenosine release in other preparations. However, in chromaffin cells or PC12 cells, preparations with great similarities to the carotid bodies, it is known that nicotinic activation induces the release of ATP (Rojas et al., 1985) and catecholamines (Courtney et al., 1991; Nagayama et al., 1999).
Although the involvement of nicotinic ACh receptors in hypoxia signalling is not a novel concept, here we demonstrate for the first time that α4 subunits present at the CB are functionally active during hypoxia and that activation of these receptors by ACh induced the production of adenosine originating mainly from extracellular catabolism of ATP by the action of ecto-5′-ectonucleotidases. These findings suggest that the excitatory effects caused by ACh in chemosensory activity include indirect activation of purinoceptors by adenosine and ATP, which strongly supports the hypothesis that ATP/adenosine are important excitatory mediators in chemotransduction.
Acknowledgments
This work was supported by FEDER/Centro de Estudos de Patologia Respiratória/Fundação para a Ciência e Tecnologia (FCT), Portugal, SV Conde is funded by a PhD grant from FCT. We are grateful to Professor Paulo Correia-de-Sá for helping in the discussion of the results and to Ms Elizabeth Halkon for reviewing the English.
Abbreviations
- AOPCP
α,β-methylene ADP
- CB
carotid body
- CSN
carotid sinus nerve
- Cyt
cytisine
- DHβE
di-hydro-β-erythroidine
- DMPP
dimethylphenylpiperazinium
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- Nic
nicotine
References
- ALEXANDER S.P., MATHIE A., PETERS J.A. Br. J. Pharmacol. 2004141(Suppl 1)S1–S126.Guide to receptors and channels [DOI] [PMC free article] [PubMed]
- BUTTIGIEG J., NURSE C.A. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem. Biophy. Res. Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- CHAMPTIAUX N., GOTTI C., CORDERO-ERAUSQUIN M., DAVID D.J., PRZYBYLSKI C., LÉNA C., CLEMENTI F., MORETTI M., LE NOVÈRE N., MCINTOSH J.M., GARDIER A.M., CHANGEUX J.P. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HAN Z.-Y., GRAILLE R., GALLEGO J., GAULTIER C., CHANGEUX J.P., LAGERCRANTZ H. β2 Nicotinic acetylcholine receptor subunit modulates protective responses to stress: A receptor basis for sleep-disordered breathing after nicotine exposure. Proc. Natl. Acad. Sci. 2002;99:13272–13277. doi: 10.1073/pnas.192463599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONDE S.V., MONTEIRO E.C. Adenosine–acetylcholine interactions at the rat carotid body. Adv. Exp. Med. Biol. 2003;536:305–311. doi: 10.1007/978-1-4419-9280-2_40. [DOI] [PubMed] [Google Scholar]
- CONDE S.V., MONTEIRO E.C. Hypoxia induces adenosine release from the rat carotid body. J. Neurochem. 2004;89:1148–1156. doi: 10.1111/j.1471-4159.2004.02380.x. [DOI] [PubMed] [Google Scholar]
- COURTNEY N.D., HOWLETT A.C., WESTFALL T.C. Regulation of nicotine-evoked dopamine release from PC12 cells. Life Sci. 1991;48:1671–1678. doi: 10.1016/0024-3205(91)90127-w. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., MILUSHEVA E., VIZI E.S., RIBEIRO J.A., SEBASTIÃO A.M. Excitatory and inhibitory effects of A1 and A2 adenosine receptor activation on the electrically evoked [3H] acetylcholine release from different areas of the rat hippocampus. J. Neurochem. 1994;63:207–214. doi: 10.1046/j.1471-4159.1994.63010207.x. [DOI] [PubMed] [Google Scholar]
- DASSO L.L.T., BUCKLER K.J., VAUGHAN-JONES R.D. Muscarinic and nicotinic receptors raises intracellular Ca2+ levels in rat carotid body type I cells. J. Physiol. 1997;498:327–338. doi: 10.1113/jphysiol.1997.sp021861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGER B., GONZALEZ C., YOSHIZAKI K., FIDONE S. Alpha-bungarotoxin binding in cat carotid body. Brain Res. 1981;205:187–193. doi: 10.1016/0006-8993(81)90731-9. [DOI] [PubMed] [Google Scholar]
- FERNÁNDEZ R., LARRAÍN C., ZAPATA P. Acute ventilatory and circulatory reactions evoked by nicotine: are they excitatory or depressant. Respir. Physiol. Neurobiol. 2002;133:173–182. doi: 10.1016/s1569-9048(02)00185-4. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R.S. Oxygen and carotid body chemotransduction: the cholinergic hypothesis – a brief history and new evaluation. Resp. Physiol. 2000;120:89–104. doi: 10.1016/s0034-5687(00)00091-8. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R.S., SHIRAHATA M., WANG H.Y., BALBIR A., CHANG I. The impact of adenosine on the release of acetylcholine, dopamine, and norepinephrine from the cat carotid body. Neurosci. Lett. 2004;367:304–308. doi: 10.1016/j.neulet.2004.06.019. [DOI] [PubMed] [Google Scholar]
- GAUDA E.B., COOPER R., JOHNSON S.M., MCLEMORE G.L., MARSHALL C. Autonomic microganglion cells: a source of acetylcholine in the rat carotid body. J. Appl. Physiol. 2004;96:384–391. doi: 10.1152/japplphysiol.00897.2003. [DOI] [PubMed] [Google Scholar]
- GAUDA E.B., NORTHINGTON F.J., LINDEN J., ROSIN D.L. Differential expression of A(2a), A(1)-adenosine and D(2)-dopamine receptor genes in rat peripheral arterial chemoreceptors during postnatal development. Brain Res. 2000;872:1–10. doi: 10.1016/s0006-8993(00)02314-3. [DOI] [PubMed] [Google Scholar]
- GONZALEZ C., ALMARAZ L., OBESO A., RIGUAL R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- HIGASHI T., MCINTOSH J.M., SHIRAHATA M. Characterization of nicotinic acetylcholine receptors in cultured arterial chemoreceptor cells of the cat. Brain Res. 2003;974:167–175. doi: 10.1016/s0006-8993(03)02574-5. [DOI] [PubMed] [Google Scholar]
- JENSEN A.A., FROLUND B., LILJEFORS T., KROGSGAARD-LARSEN P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., CONFORTI L., MILLHORN D.E. Gene expression and function of adenosine A(2A) receptor in the rat carotid body. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:273–282. doi: 10.1152/ajplung.2000.279.2.L273. [DOI] [PubMed] [Google Scholar]
- MAXWELL D.L., FULLER R.W., CONRADSON T.-B., DIXON C.M.S., ABER V., HUGHES M.B., BARNES P.J. Contrasting effects of two xanthines, theophyline and enprofylline, on the cardio-respiratory stimulation of infused adenosine in man. Acta Physiol. Scan. 1987;131:459–465. doi: 10.1111/j.1748-1716.1987.tb08262.x. [DOI] [PubMed] [Google Scholar]
- MAXWELL D.L., FULLER R.W., NOLOP K.B., DIXON C.M.S., HUGHES M.B. Effects of adenosine on ventilatory responses to hypoxia and hypercapnia in humans. J. Appl. Physiol. 1986;61:1762–1766. doi: 10.1152/jappl.1986.61.5.1762. [DOI] [PubMed] [Google Scholar]
- MCQUEEN D.S. A quantitative study of the effects of cholinergic drugs on carotid chemoreceptors in the cat. J. Physiol. (London) 1977;273:515–532. doi: 10.1113/jphysiol.1977.sp012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCQUEEN D.S.Pharmacological aspects of putative transmitters in the carotid body Physiology of the Peripheral Arterial Chemoreceptors 1983Amsterdam: Elsevier Science; 149–155.ed. Acker, H. & O'Regan, R.G., pp [Google Scholar]
- MCQUEEN D.S., RIBEIRO J.A. Effect of adenosine on carotid chemoreceptor activity in the cat. Br. J. Pharmacol. 1981;74:129–136. doi: 10.1111/j.1476-5381.1981.tb09964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCQUEEN D.S., RIBEIRO J.A. On the specificity and type of receptor involved in carotid body chemoreceptor activation by adenosine in the cat. Br. J. Pharmacol. 1983;80:347–354. doi: 10.1111/j.1476-5381.1983.tb10040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCQUEEN D.S., RIBEIRO J.A. Pharmacological characterization of the receptor involved in chemoexcitation induced by adenosine. Br. J. Pharmacol. 1986;88:615–620. doi: 10.1111/j.1476-5381.1986.tb10242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEGHJI P., BURNSTOCK G. Inhibition of extracellular ATP degradation in endothelial cells. Life Sci. 1995;57:763–771. doi: 10.1016/0024-3205(95)02004-3. [DOI] [PubMed] [Google Scholar]
- MONTEIRO E.C., RIBEIRO J.A. Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Naunyn Schmiedeberg's Arch. Pharmacol. 1987;335:143–148. doi: 10.1007/BF00177715. [DOI] [PubMed] [Google Scholar]
- MONTEIRO E.C., VERA-CRUZ P., MONTEIRO T.C., SILVA E SOUSA M.A. Adenosine increases the cAMP content of the rat carotid body in vitro. Adv. Exp. Med. Biol. 1996;410:299–303. doi: 10.1007/978-1-4615-5891-0_45. [DOI] [PubMed] [Google Scholar]
- NAGAYAMA T., MATSUMOTO T., KUWAKUBO F., FUKUSHIMA Y., YOSHIDA M., SUZUKI-KUSABA M., HISA H., KIMURA T., SATOH S. Role of calcium channels in catecholamine secretion in the rat adrenal gland. J. Physiol. 1999;520:503–512. doi: 10.1111/j.1469-7793.1999.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBESO A., GOMEZ-NINO M.A., ALMARAZ L., DINGER B., FIDONE S., GONZALEZ C. Evidence for two types of nicotinic receptors in the cat carotid body chemoreceptor cells. Brain Res. 1997;754:298–302. doi: 10.1016/s0006-8993(97)00185-6. [DOI] [PubMed] [Google Scholar]
- RIBEIRO J.A., MONTEIRO E.C. On the adenosine receptor involved in the excitatory action of adenosine on respiration: antagonist profile. Nucleosides Nucleotides. 1991;10:945–953. [Google Scholar]
- RIBEIRO J.A., CUNHA R.A., CORREIA-DE-SÁ P., SEBASTIÃO A. Purinergic regulation of acetylcholine release. Prog. Brain Res. 1996;109:231–241. doi: 10.1016/s0079-6123(08)62107-x. [DOI] [PubMed] [Google Scholar]
- ROJAS E., POLLARD H.B., HELDMAN E. Real-time measurements of acetylcholine-induced release of ATP from bovine medullary chromaffin cells. FEBS Lett. 1985;185:323–327. doi: 10.1016/0014-5793(85)80931-5. [DOI] [PubMed] [Google Scholar]
- RONG W., GOURINE A.V., COCKAYNE D.A., XIANG Z., FORD A.P.D.W., SPYER M., BURNSTOCK G. Pivotal role of Nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J. Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAHATA M., ISHIZAWA Y., RUDISILL M., SCHOFIELD B., FITZGERALD R.S. Presence of nicotinic acetylcholine receptors in cat carotid body afferent system. Brain Res. 1998;814:213–217. doi: 10.1016/s0006-8993(98)01015-4. [DOI] [PubMed] [Google Scholar]
- UEMATSU T., KOZAWA O., MATSUNO H., NIWA M., YOSHIKOSHI H., OH-UCHI M., CONO K., NAGASHIMA S., KANAMARU M. Pharmacokinetics and tolerability of intravenous infusión of adenosine (SUNY4001) in healthy volunteers. Br. J. Clin. Pharmacol. 2000;50:177–181. doi: 10.1046/j.1365-2125.2000.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATT A.H., ROUTLEDGE P.A. Adenosine stimulates respiration in man. Br. J. Pharmacol. 1985;20:503–506. doi: 10.1111/j.1365-2125.1985.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATT A.H., REID P.G., STEPHENS M.R., ROUTLEDGE P.A. Adenosine-induced respiratory stimulation in man depends on site of infusion. Evidence for an action on the carotid body. Br. J. Clin. Pharmacol. 1987;23:486–490. doi: 10.1111/j.1365-2125.1987.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG M., ZHONG H., VOLLMER C., NURSE C.A. Co-release of ATP and ACh mediates hypoxic signaling at rat carotid body chemoreceptors. J. Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWART R., VAN KLEEF R.G.D.M., GOTTI C., SMULDERS C.J.G.M., VIJERVERBERG H.P.M. Competitive potentiation of acetylcholine effects on neuronal nicotinic receptors by acetylcholinesterase-inhibiting drugs. J. Neurochem. 2000;75:2492–2500. doi: 10.1046/j.1471-4159.2000.0752492.x. [DOI] [PubMed] [Google Scholar]