Abstract
OBJECTIVES—To examine the effects of ceramide, which is a lipid second messenger of cell surface receptors, including tumour necrosis factor α (TNFα), interleukin 1 (IL1), and Fas receptors, on rheumatoid arthritis (RA) synovial cells. METHODS—Synovial cells from RA patients and normal skin fibroblasts were cultured with cell permeable ceramide (C2-ceramide). Apoptosis was assessed by microscopic observation of morphological changes, nuclear staining, and DNA electrophoresis. DNA synthesis was examined by thymidine incorporation. RESULTS—C2-ceramide induced reversible morphological changes of synovial cells such as cell rounding within four hours. Subsequently, irreversible nuclear changes characteristic to apoptosis were observed at 48 hours. DNA synthesis was not promoted. The addition of ceramide exerted similar effects on cultured dermal fibroblasts. CONCLUSION—Ceramide induced apoptosis in RA synovial cells. Ceramide could be a second messenger specific for apoptosis of RA synovial cells. Keywords: ceramide; apoptosis; rheumatoid arthritis
Full Text
The Full Text of this article is available as a PDF (170.2 KB).
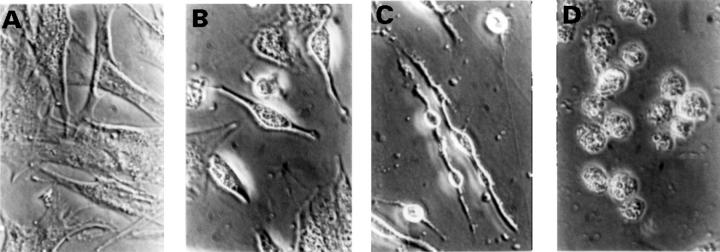
Figure 1 .
Induction of cell death in RA synovial cells by C2-ceramide. RA synovial cells were incubated with 10 µM of C2-ceramide in serum free RPMI 1640 medium (supplemented with 40 ng/ml PDGF) for 0 hours (A), 4 hours (B), 24 hours (C), and 48 hours (D). Cells were observed by phase contrast microscopy (original magnification × 400).
Figure 2 .
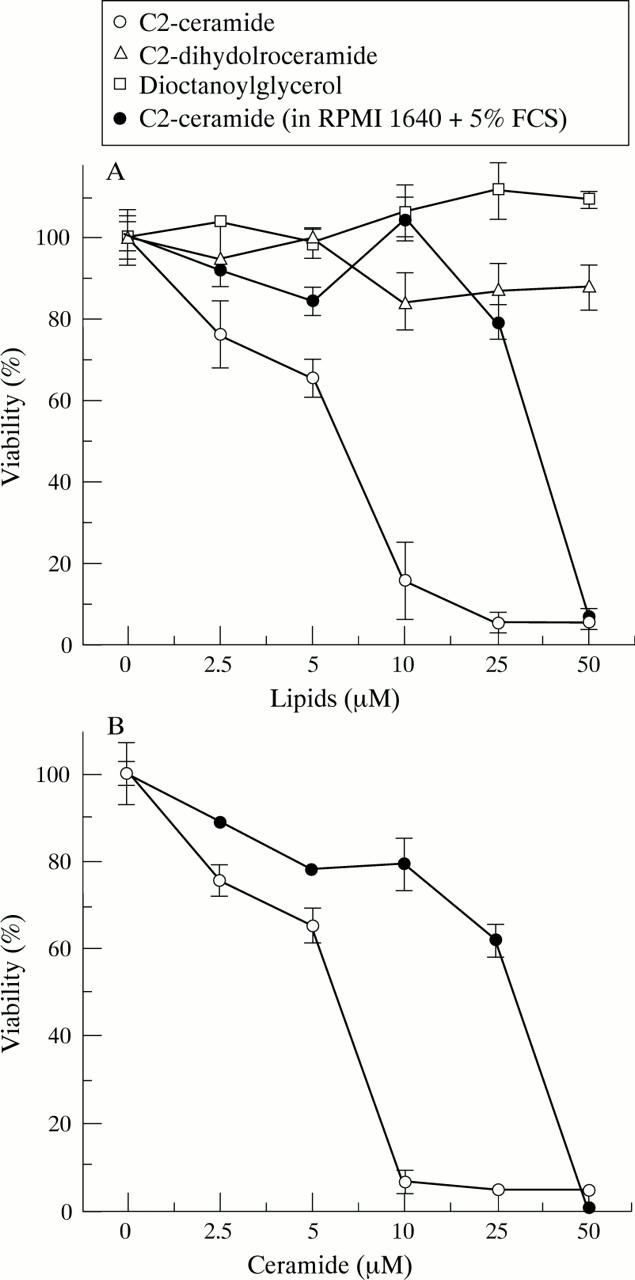
Cytotoxicity of C2-ceramide on RA synovial cells and dermal fibroblasts. RA synovial cells (A) and normal dermal fibroblasts (B) were treated with C2-ceramide, C2-dihydroceramide, and dioctanoylglycerol in serum free RPMI 1640 medium (supplemented with 40 ng/ml PDGF), C2-ceramide in RPMI 1640 containing 5% FCS for 48 hours. Cell viability was determined by crystal violet assay. Values are the mean (SD) of triplicate cultures. The data were representative of synovial cells from eight RA patients and dermal fibroblasts from normal skin of three controls.
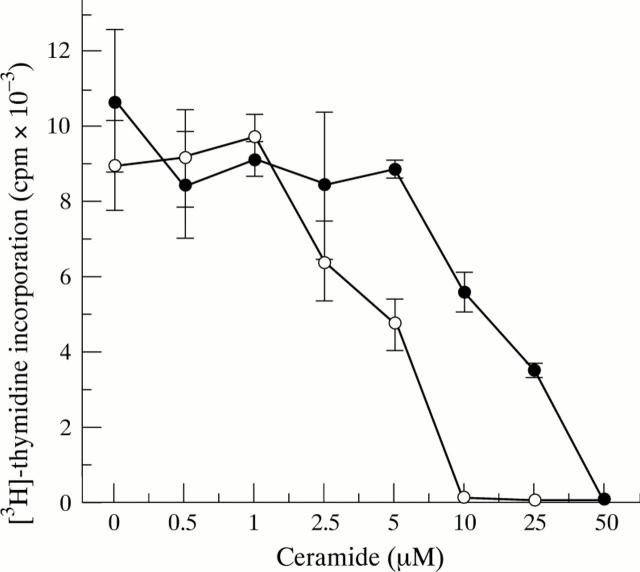
Figure 3 .
Inhibitory effect of ceramide on synovial cell DNA synthesis. RA synovial cells were treated with C2-ceramide in serum free RPMI 1640 medium (supplemented with 40 ng/ml PDGF, open symbols) or in RPMI 1640 containing 5% FCS (closed symbols) for 48 hours. DNA synthesis was determined by 3H-thymidine incorporation assay. Values are the mean (SD) of triplicate cultures. The data were representative of synovial cell samples from eight RA patients.
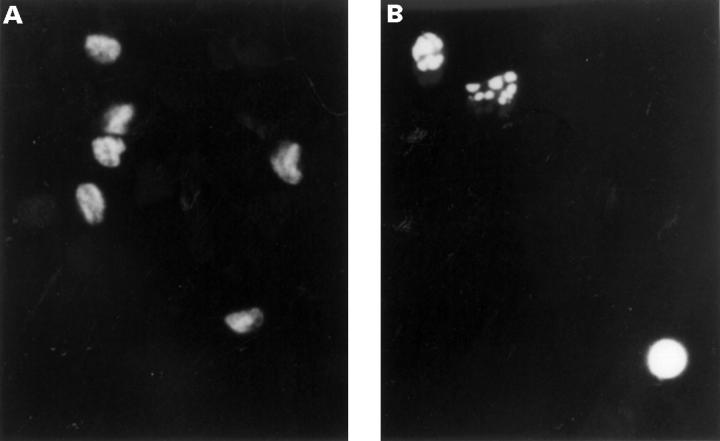
Figure 4 .
Nuclear condensation and fragmentation of RA synovial cells induced by C2-ceramide. RA synovial cells were treated with 0.1% ethanol, as a control (A) or 10 µM C2-ceramide (B) for 48 hours. Nuclei were stained with Hoechst 33258 and observed by fluorescence microscopy (original magnification × 400).
Figure 5 .
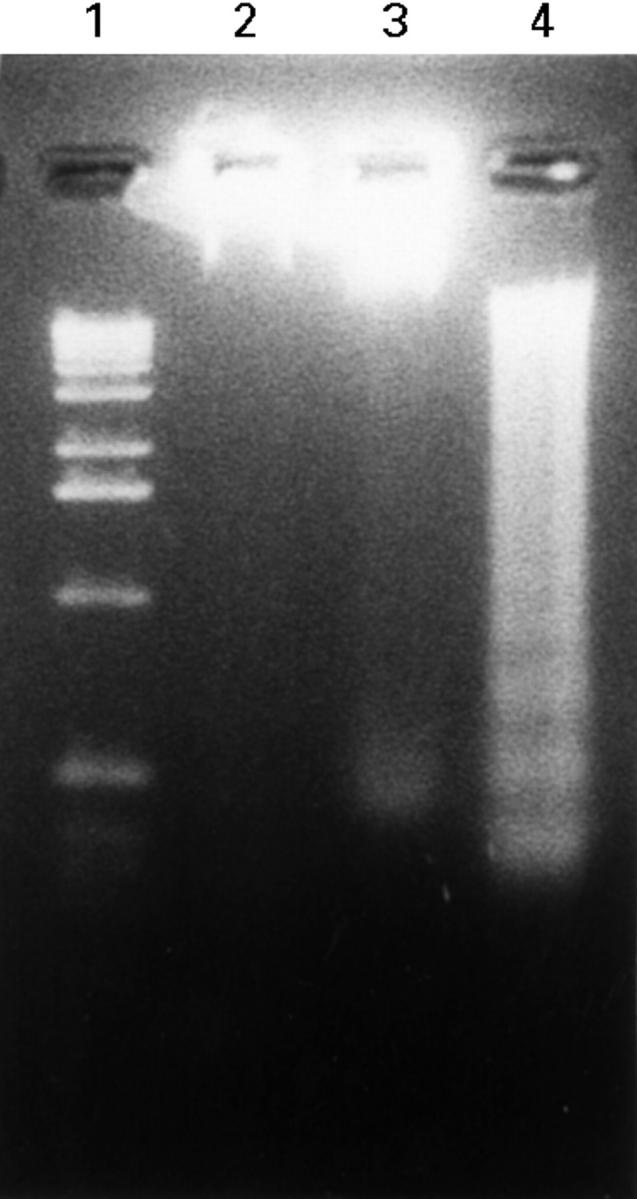
DNA fragmentation of ceramide treated RA synovial cells. RA synovial cells were treated with 10 µM C2-ceramide for the indicated periods. Extracted DNA was electrophoresed on a 2% agarose gel, and stained with ethidium bromide. Lane 1:1kb ladder DNA molecular weight marker, lane 2:0.1% ethanol, lane 3:C2-ceramide for 24 hours, lane 4:C2-ceramide for 48 hours.
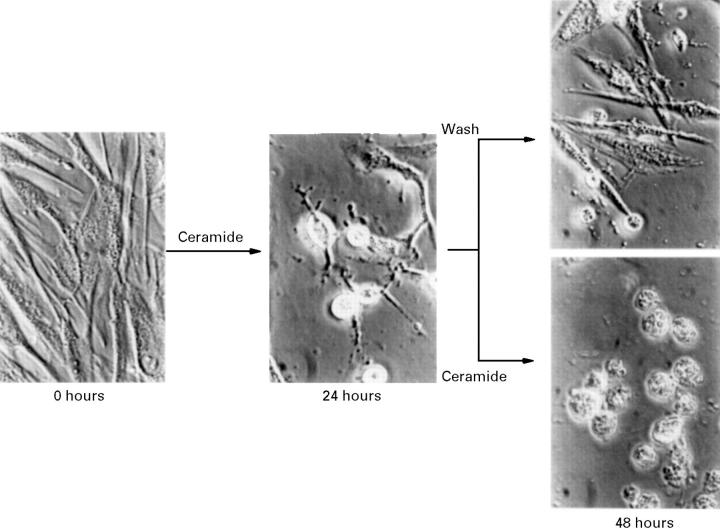
Figure 6 .
Reversibility of early morphological changes induced by ceramide. RA synovial cells were incubated with 10 µM of C2-ceramide in serum free RPMI 1640 medium (supplemented with 40 ng/ml PDGF) for 24 hours. The medium was changed to ceramide free medium, and the culture was continued for additional 24 hours. The cells were observed by phase contrast microscopy (original magnification × 400).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Ballou L. R., Chao C. P., Holness M. A., Barker S. C., Raghow R. Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem. 1992 Oct 5;267(28):20044–20050. [PubMed] [Google Scholar]
- Bielawska A., Crane H. M., Liotta D., Obeid L. M., Hannun Y. A. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993 Dec 15;268(35):26226–26232. [PubMed] [Google Scholar]
- Boucher L. M., Wiegmann K., Fütterer A., Pfeffer K., Machleidt T., Schütze S., Mak T. W., Krönke M. CD28 signals through acidic sphingomyelinase. J Exp Med. 1995 Jun 1;181(6):2059–2068. doi: 10.1084/jem.181.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Benedetti M., Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995 Nov 1;14(21):5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. M., Leizer T., Hamilton J. A. Stimulation of human synovial fibroblast DNA synthesis by platelet-derived growth factor and fibroblast growth factor. Differences to the activation by IL-1. J Immunol. 1989 May 1;142(9):3098–3103. [PubMed] [Google Scholar]
- Butler D. M., Piccoli D. S., Hart P. H., Hamilton J. A. Stimulation of human synovial fibroblast DNA synthesis by recombinant human cytokines. J Rheumatol. 1988 Oct;15(10):1463–1470. [PubMed] [Google Scholar]
- Cao Z., Xiong J., Takeuchi M., Kurama T., Goeddel D. V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996 Oct 3;383(6599):443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Chan G., Ochi A. Sphingomyelin-ceramide turnover in CD28 costimulatory signaling. Eur J Immunol. 1995 Jul;25(7):1999–2004. doi: 10.1002/eji.1830250730. [DOI] [PubMed] [Google Scholar]
- Cifone M. G., Roncaioli P., De Maria R., Camarda G., Santoni A., Ruberti G., Testi R. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995 Dec 1;14(23):5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbaibo G. S., Obeid L. M., Hannun Y. A. Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. J Biol Chem. 1993 Aug 25;268(24):17762–17766. [PubMed] [Google Scholar]
- Dobrowsky R. T., Werner M. H., Castellino A. M., Chao M. V., Hannun Y. A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994 Sep 9;265(5178):1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa K., Aono H., Hasunuma T., Yamamoto K., Mita S., Nishioka K. Activation of transcription factor NF-kappa B in human synovial cells in response to tumor necrosis factor alpha. Arthritis Rheum. 1996 Feb;39(2):197–203. doi: 10.1002/art.1780390205. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Bissonnette R., Mahboubi A., Martin S., Nishioka W., Brunner T., Baier G., Baier-Bitterlich G., Byrd C., Lang F. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995 Apr;2(4):341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Butler D. M., Stanton H. Cytokine interactions promoting DNA synthesis in human synovial fibroblasts. J Rheumatol. 1994 May;21(5):797–803. [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hasunuma T., Hoa T. T., Aono H., Asahara H., Yonehara S., Yamamoto K., Sumida T., Gay S., Nishioka K. Induction of Fas-dependent apoptosis in synovial infiltrating cells in rheumatoid arthritis. Int Immunol. 1996 Oct;8(10):1595–1602. doi: 10.1093/intimm/8.10.1595. [DOI] [PubMed] [Google Scholar]
- Hauser J. M., Buehrer B. M., Bell R. M. Role of ceramide in mitogenesis induced by exogenous sphingoid bases. J Biol Chem. 1994 Mar 4;269(9):6803–6809. [PubMed] [Google Scholar]
- Jayadev S., Liu B., Bielawska A. E., Lee J. Y., Nazaire F., Pushkareva MYu, Obeid L. M., Hannun Y. A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995 Feb 3;270(5):2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Ord T., Testa M. P., Zhong L. T., Bredesen D. E. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2234–2238. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kolesnick R., Fuks Z. Ceramide: a signal for apoptosis or mitogenesis? J Exp Med. 1995 Jun 1;181(6):1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T. J., Kirschner M. W., Koths K., Kwiatkowski D. J. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997 Oct 10;278(5336):294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Kumkumian G. K., Lafyatis R., Remmers E. F., Case J. P., Kim S. J., Wilder R. L. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J Immunol. 1989 Aug 1;143(3):833–837. [PubMed] [Google Scholar]
- Laulederkind S. J., Bielawska A., Raghow R., Hannun Y. A., Ballou L. R. Ceramide induces interleukin 6 gene expression in human fibroblasts. J Exp Med. 1995 Aug 1;182(2):599–604. doi: 10.1084/jem.182.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T., Naito M., Fujita N., Noguchi K., Tsuruo T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun. 1995 Dec 26;217(3):1185–1192. doi: 10.1006/bbrc.1995.2894. [DOI] [PubMed] [Google Scholar]
- Mathias S., Younes A., Kan C. C., Orlow I., Joseph C., Kolesnick R. N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993 Jan 22;259(5094):519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Koike R., Kohsaka H., Kushi Y., Handa S., Yagita H., Miyasaka N. Ceramide induces apoptosis via CPP32 activation. FEBS Lett. 1996 Oct 21;395(2-3):267–271. doi: 10.1016/0014-5793(96)01050-2. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Wu J., Cheng J., Zhou T. Autoimmune disease. A problem of defective apoptosis. Arthritis Rheum. 1994 Oct;37(10):1415–1420. doi: 10.1002/art.1780371002. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Aono H., Hasunuma T., Yamamoto K., Shirai T., Hirohata K., Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995 Apr;38(4):485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Olivera A., Buckley N. E., Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992 Dec 25;267(36):26121–26127. [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V. M., Goeddel D. V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995 Sep 8;269(5229):1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Rudel T., Bokoch G. M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997 Jun 6;276(5318):1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Santana P., Peña L. A., Haimovitz-Friedman A., Martin S., Green D., McLoughlin M., Cordon-Cardo C., Schuchman E. H., Fuks Z., Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996 Jul 26;86(2):189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tepper C. G., Jayadev S., Liu B., Bielawska A., Wolff R., Yonehara S., Hannun Y. A., Seldin M. F. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Kampen E., Himmler A., Machleidt T., Krönke M. Human 55-kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Biol Chem. 1992 Sep 5;267(25):17997–18001. [PubMed] [Google Scholar]
- Yanaga F., Watson S. P. Ceramide does not mediate the effect of tumour necrosis factor alpha on superoxide generation in human neutrophils. Biochem J. 1994 Mar 15;298(Pt 3):733–738. doi: 10.1042/bj2980733. [DOI] [PMC free article] [PubMed] [Google Scholar]