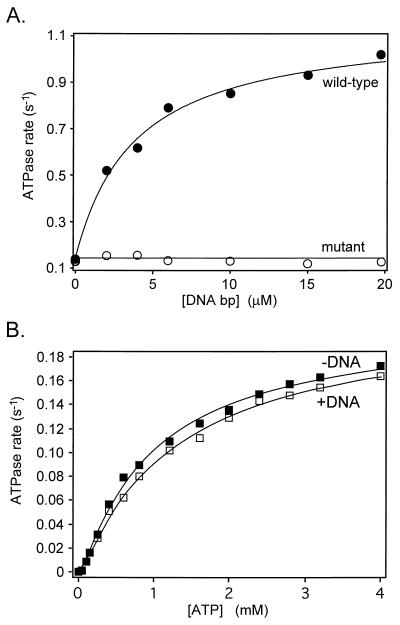
Figure 4.
ATPase activity of wild-type gyrase and an active-site mutant. (A) DNA binding to the active-site mutant (A2Ser122B2) is unable to stimulate the rate of ATP hydrolysis. Reactions contained 20 nM gyrase, 2 mM ATP, and the indicated amounts of linear pBR322 DNA; rates were measured at 25°C. (B) The kinetics of ATP hydrolysis by A2Ser122B2. Samples contained 20 nM enzyme and 5 nM linear pBR322 DNA, where indicated. Reactions were carried out at 25°C. The data were fitted to the model described by Kampranis and Maxwell (42).