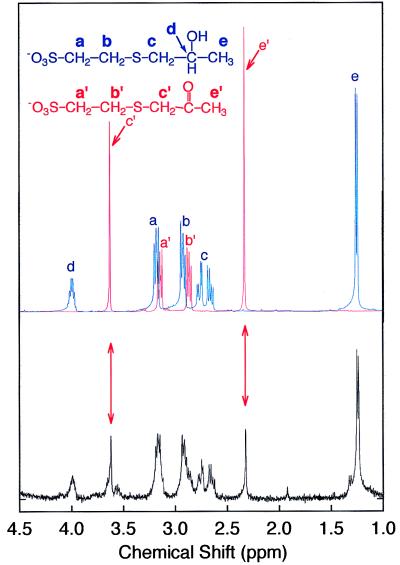
Figure 3.
1H NMR spectrum of the product of component III- and IV-catalyzed reaction of NAD+ and 2-hydroxypropyl–CoM. The spectra of chemically synthesized 2-hydroxypropyl–CoM (in blue) and 2-ketopropyl–CoM (in red) are overlaid and shown directly above the product spectrum. Resonances at 1.23 and 3.98 ppm correspond to methyl group (e) and proton (d) of 2-hydroxypropyl–CoM, respectively. Resonances at 2.63 and 2.74 ppm correspond to methylene group (c) of 2-hydroxypropyl–CoM. The multiplets at 2.89 and 3.15 ppm are the overlapping resonances of methylene groups (b, b′) and (a, a′) of 2-hydroxypropyl–CoM and 2-ketopropyl–CoM, respectively. The singlet at 3.63 ppm corresponds to the (c′) methylene group of 2-ketopropyl–CoM, which integrates to two protons. The signal at 2.33 ppm corresponds to methyl group (e′) of 2-ketopropyl–CoM, which integrates to three protons.