Figure 3.
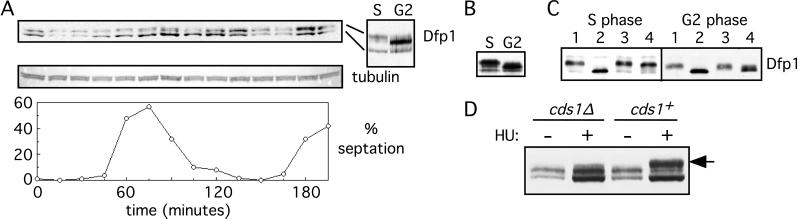
Expression and phosphorylation of Dfp1 during cell cycle progression. (A) A culture of the strain GBY398 was blocked in G2 and then released synchronously into the cell cycle. The culture was sampled every 15 min, and extracts were prepared. Dfp1 was detected by immunoblot analysis. The blot was reprobed for tubulin as a loading control. Synchrony was assessed by measuring the percentage of cells with a septum (% septation). The 60- (S) and 135-min (G2) samples are also shown side by side for comparison of the different Dfp1 isoforms. (B) Extracts were prepared from cells blocked in S phase (GBY397) or in G2 phase (GBY398) by using denaturing conditions. Proteins were immunoblotted, probing for Dfp1. (C) Native extracts were prepared from cells blocked in S or G2 phase. Hsk1 and associated Dfp1 were immunoprecipitated, and the immunoprecipitates were incubated with λ phosphatase (lanes 2), without λ phosphatase (lanes 3), or with λ phosphatase plus the inhibitor vanadate (lanes 4). The untreated controls are in lanes 1. Reaction products were subjected to immunoblot analysis, probing for Dfp1. Note that the fastest-migrating Dfp1 isoform seen in A and B is extracted inefficiently under native conditions. (D) The phosphorylation state of Dfp1 in S phase cells was analyzed in cds1+ or cds1Δ strains. Samples were taken from cultures arrested in S phase with hydroxyurea (+HU) or from asynchronous cultures (−HU). The position of the S phase Dfp1 phosphoisomer is indicated by the arrow.