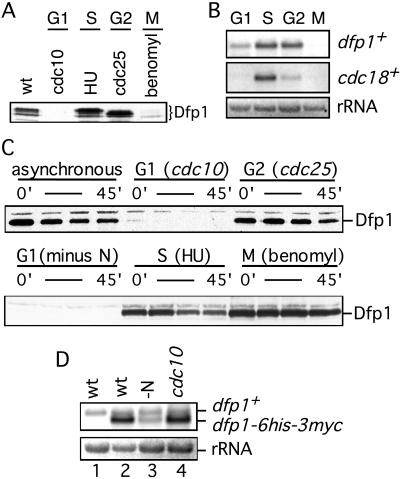
Figure 4.
Dfp1 expression is down-regulated during G1 phase. (A) Dfp1 levels in cells blocked in G1 (GBY401), S (GBY397), G2 (GBY398), or M (GBY397) phases were compared with levels in asynchronous culture (wt, GBY397) by using immunoblot analysis. The Dfp1 species are indicated. Equal loading of all lanes was confirmed by reprobing immunoblots with anti-tubulin antibody (not shown). (B) Total RNA was prepared from cells blocked as in A and fractionated on formaldehyde-agarose gels. A Northern blot was probed for dfp1+ mRNA and cdc18+ mRNA. The ethidium bromide-stained rRNA is shown as a control for equal loading. (C) Strains (GBY410, 411, 412) expressing dfp1–6his-3myc from the repressible nmt1 promoter were blocked at the indicated cell cycle positions. Expression of dfp1–6his-3myc was repressed by the addition of thiaminee, and Dfp1 protein levels at 0, 15, 30, and 45 min after repression was analyzed by immunoblotting. Equal loading of all lanes was confirmed by reprobing immunoblots with anti-tubulin antibody (not shown). (D) RNA was prepared from a logarithmically growing culture of wild-type cells (wt, lane 1), from GBY410 in logarithmically growing culture (wt, lane 2), from GBY410 cultured in the absence of nitrogen for 4 hr (-N, lane 3), or from GBY411 cultured at 36°C for 4 hr (cdc10, lane 4). A Northern blot of this RNA was probed with the dfp1+ ORF. The positions of the dfp1+ and dfp1–6his-3myc mRNAs are indicated. The ethidium bromide-stained rRNA is shown as a control for equal loading.