Full Text
The Full Text of this article is available as a PDF (442.9 KB).
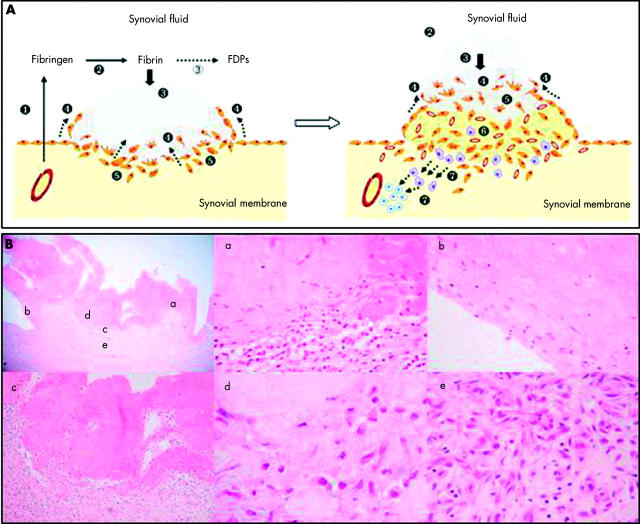
Figure 1 .
Pathogenesis of RA as a fibrin induced disease. (A) The figure shows two sequential panels; the numbers indicate the pathogenic steps leading to established RA. 1. Exudation of fibrinogen and clotting factors to the joint space follows joint swelling. 2. Haemostasis activation within the cavity leads to fibrin formation. 3. Fibrin clots are partially removed by the plasminogen system (unfilled circle), but most of them get stuck to the synovial intima (black circle). 4. Cells at the fibrin-synovium interface migrate into and around the deposits (arrows). 5. Clot components induce multiple activating pathways in synoviocytes by the coupling of specific receptors. These include proliferation, secretion of proteinases, and synthesis of proinflammatory mediators. 6. A fibroproliferative tissue appears underneath the front of migrating cells, as a result of remodelling of the invaded clots by activated cells. In this area, macrophages and blood vessels are increased owing to the release of growth factors and chemokines. 7. Remodelling induces modifications in the structure of fibrin chains, which become immunogenic. Epitopes from these transformed autologous peptides are presented to T lymphocytes, which in turn initiate a specific immune reactivity against them. As illustrated in the right panel, continuous deposition of fibrin clots and the advance of the front of migration account for tissue hypertrophic growth at the areas of attachment. (B) Left upper panel: photomicrograph of a rheumatoid synovial membrane stained with haematoxylin-eosin (x40). The image shows a large eosinophilic deposit of fibrin adhering to the synovial tissue. Areas indicated with the letters a to e are magnified in the following panels. (a) A detail of cell migration into the deposit (x200). (b) The deposit shows a partial epithelisation at the margin of attachment with the tissue (x400). (c) The interface area is magnified (x100) to see the sharp differences of microarchitecture and cellularity at both sides. (d) Over the interface shown in (c), cells are scattered and have various shapes and sizes. Some of them are undergoing mitosis. The interstitium is predominantly amorphous (x1000). (e) At the base of the clot, the tissue looks fibrotic, hypercellular with fibroblasts and mononuclear cells, and rich in microvasculature. Some fibroblasts are undergoing mitosis. FDPs, fibrin(ogen) degradation peptides.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. B., Gormsen J. Fibrinolytic and fibrin stabilizing activity of synovial membranes. Ann Rheum Dis. 1970 May;29(3):287–293. doi: 10.1136/ard.29.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berckmans René J., Nieuwland Rienk, Tak Paul Peter, Böing Anita N., Romijn Fred P. H. T. M., Kraan Maarten C., Breedveld Ferdinand C., Hack C. Erik, Sturk Augueste. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002 Nov;46(11):2857–2866. doi: 10.1002/art.10587. [DOI] [PubMed] [Google Scholar]
- Bini A., Wu D., Schnuer J., Kudryk B. J. Characterization of stromelysin 1 (MMP-3), matrilysin (MMP-7), and membrane type 1 matrix metalloproteinase (MT1-MMP) derived fibrin(ogen) fragments D-dimer and D-like monomer: NH2-terminal sequences of late-stage digest fragments. Biochemistry. 1999 Oct 19;38(42):13928–13936. doi: 10.1021/bi991096g. [DOI] [PubMed] [Google Scholar]
- Braat E. A., Jie A. F., Ronday H. K., Beekman B., Rijken D. C. Urokinase-mediated fibrinolysis in the synovial fluid of rheumatoid arthritis patients may be affected by the inactivation of single chain urokinase type plasminogen activator by thrombin. Ann Rheum Dis. 2000 Apr;59(4):315–318. doi: 10.1136/ard.59.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso Nathalie, Hamilton John A. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002 Sep;46(9):2268–2279. doi: 10.1002/art.10498. [DOI] [PubMed] [Google Scholar]
- Carmassi F., de Negri F., Morale M., Song K. Y., Chung S. I. Fibrin degradation in the synovial fluid of rheumatoid arthritis patients: a model for extravascular fibrinolysis. Semin Thromb Hemost. 1996;22(6):489–496. doi: 10.1055/s-2007-999049. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Biomedicine. Clotting factors build blood vessels. Science. 2001 Aug 31;293(5535):1602–1604. doi: 10.1126/science.1064981. [DOI] [PubMed] [Google Scholar]
- Caughey D. E., Highton T. C. Components of the fibrinolytic system in synovial joints. Normal bovine compared with normal and abnormal human synovial joints. Ann Rheum Dis. 1967 Jul;26(4):297–305. doi: 10.1136/ard.26.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino G., Cicala C., Bucci M., Sorrentino L., Ambrosini G., DeDominicis G., Altieri D. C. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997 May 15;99(10):2446–2451. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen I., Donde R., Andersen R. B. The primary plasmin inhibitor in rheumatoid synovial fluid. Arthritis Rheum. 1977 Sep-Oct;20(7):1354–1358. doi: 10.1002/art.1780200709. [DOI] [PubMed] [Google Scholar]
- Clemmensen I., Hølund B., Andersen R. B. Fibrin and fibronectin in rheumatoid synovial membrane and rheumatoid synovial fluid. Arthritis Rheum. 1983 Apr;26(4):479–485. doi: 10.1002/art.1780260405. [DOI] [PubMed] [Google Scholar]
- Corbett S. A., Lee L., Wilson C. L., Schwarzbauer J. E. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997 Oct 3;272(40):24999–25005. doi: 10.1074/jbc.272.40.24999. [DOI] [PubMed] [Google Scholar]
- Corbett S. A., Wilson C. L., Schwarzbauer J. E. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996 Jul 1;88(1):158–166. [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Fassbender H. G., Gay S. Synovial processes in rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;76:1–7. doi: 10.3109/03009748809102945. [DOI] [PubMed] [Google Scholar]
- Fibbi G., Pucci M., Serni U., Cerinic M. M., Del Rosso M. Antisense targeting of the urokinase receptor blocks urokinase-dependent proliferation, chemoinvasion, and chemotaxis of human synovial cells and chondrocytes in vitro. Proc Assoc Am Physicians. 1998 Jul-Aug;110(4):340–350. [PubMed] [Google Scholar]
- Firestein Gary S., Zvaifler Nathan J. How important are T cells in chronic rheumatoid synovitis?: II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002 Feb;46(2):298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- Furmaniak-Kazmierczak E., Cooke T. D., Manuel R., Scudamore A., Hoogendorn H., Giles A. R., Nesheim M. Studies of thrombin-induced proteoglycan release in the degradation of human and bovine cartilage. J Clin Invest. 1994 Aug;94(2):472–480. doi: 10.1172/JCI117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Marzo-Ortega H., McGonagle D., Wakefield R., Proudman S., Conaghan P., Gooi J., Emery P. Persistence of mild, early inflammatory arthritis: the importance of disease duration, rheumatoid factor, and the shared epitope. Arthritis Rheum. 1999 Oct;42(10):2184–2188. doi: 10.1002/1529-0131(199910)42:10<2184::AID-ANR20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hotary Kevin B., Yana Ikuo, Sabeh Farideh, Li Xiao-Yan, Holmbeck Kenn, Birkedal-Hansen Henning, Allen Edward D., Hiraoka Nobuaki, Weiss Stephen J. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002 Feb 4;195(3):295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Hirata S., Andoh Y., Kubo H., Nakagawa N., Nishibayashi Y., Mizuno K. An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Rheumatol Int. 1996;16(2):53–60. doi: 10.1007/BF01816436. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Saari H., Santavirta S., Antti-Poika I., Sorsa T., Nykänen P., Kemppinen P. Synovial fibroblasts. Scand J Rheumatol Suppl. 1988;76:95–103. doi: 10.3109/03009748809102958. [DOI] [PubMed] [Google Scholar]
- Kummer J. A., Abbink J. J., de Boer J. P., Roem D., Nieuwenhuys E. J., Kamp A. M., Swaak T. J., Hack C. E. Analysis of intraarticular fibrinolytic pathways in patients with inflammatory and noninflammatory joint diseases. Arthritis Rheum. 1992 Aug;35(8):884–893. doi: 10.1002/art.1780350806. [DOI] [PubMed] [Google Scholar]
- Liu X., Piela-Smith T. H. Fibrin(ogen)-induced expression of ICAM-1 and chemokines in human synovial fibroblasts. J Immunol. 2000 Nov 1;165(9):5255–5261. doi: 10.4049/jimmunol.165.9.5255. [DOI] [PubMed] [Google Scholar]
- Masson-Bessière C., Sebbag M., Girbal-Neuhauser E., Nogueira L., Vincent C., Senshu T., Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001 Mar 15;166(6):4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- Ronday H. K., Smits H. H., Van Muijen G. N., Pruszczynski M. S., Dolhain R. J., Van Langelaan E. J., Breedveld F. C., Verheijen J. H. Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1996 May;35(5):416–423. doi: 10.1093/rheumatology/35.5.416. [DOI] [PubMed] [Google Scholar]
- Rothschild B. M., Thompson L. D., Pifer D. D., Chesney C. M. Perturbation of protease inhibitors and substrates in inflammatory arthritis. Semin Thromb Hemost. 1985 Oct;11(4):394–404. doi: 10.1055/s-2007-1004400. [DOI] [PubMed] [Google Scholar]
- Sarkissian M., Lafyatis R. Integrin engagement regulates proliferation and collagenase expression of rheumatoid synovial fibroblasts. J Immunol. 1999 Feb 1;162(3):1772–1779. [PubMed] [Google Scholar]
- Schellekens G. A., de Jong B. A., van den Hoogen F. H., van de Putte L. B., van Venrooij W. J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998 Jan 1;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Almond T. J., Walton K. W., Hunneyball I. M. The role of fibronectin in the pathogenesis of antigen-induced arthritis in the rabbit. J Pathol. 1983 Oct;141(2):143–156. doi: 10.1002/path.1711410205. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Walton K. W. The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981 Aug;62(4):362–368. [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Felsenfeld D. P., Galbraith C. G. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998 Feb;8(2):51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- Shin H., Kitajima I., Nakajima T., Shao Q., Tokioka T., Takasaki I., Hanyu N., Kubo T., Maruyama I. Thrombin receptor mediated signals induce expressions of interleukin 6 and granulocyte colony stimulating factor via NF-kappa B activation in synovial fibroblasts. Ann Rheum Dis. 1999 Jan;58(1):55–60. doi: 10.1136/ard.58.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Nakajima T., Kitajima I., Shigeta K., Abeyama K., Imamura T., Okano T., Kawahara K., Nakamura T., Maruyama I. Thrombin receptor-mediated synovial proliferation in patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1995 Sep;76(3 Pt 1):225–233. doi: 10.1006/clin.1995.1120. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute O., López-Armada M. J., Calvo E., Díez-Ortego I., Largo R., Egido J., Herrero-Beaumont G. Fibrin generated in the synovial fluid activates intimal cells from their apical surface: a sequential morphological study in antigen-induced arthritis. Rheumatology (Oxford) 2003 Jan;42(1):19–25. doi: 10.1093/rheumatology/keg021. [DOI] [PubMed] [Google Scholar]
- Van De Putte L. B., Hegt V. N., Overbeek T. E. Activators and inhibitors of fibrinolysis in rheumatoid and nonrheumatoid synovial membranes. A histochemical study. Arthritis Rheum. 1977 Mar;20(2):671–678. doi: 10.1002/art.1780200206. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Pippen A. M., Greenberg C. S. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991 Aug;34(8):996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- Zacharski L. R., Brown F. E., Memoli V. A., Kisiel W., Kudryk B. J., Rousseau S. M., Hunt J. A., Dunwiddie C., Nutt E. M. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992 May;63(2):155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]