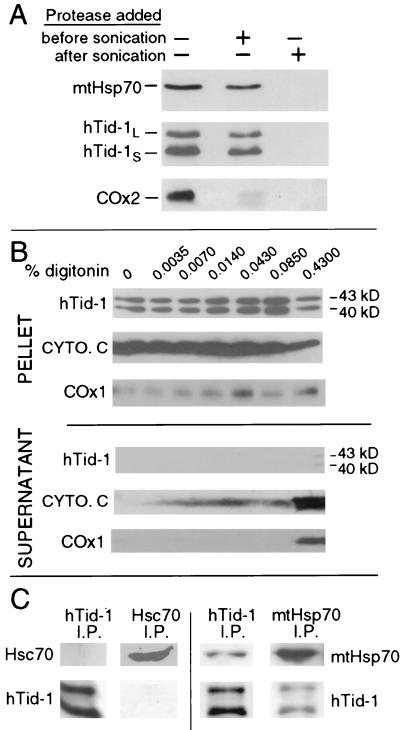
Figure 2.
hTid-1L and hTid-1S are localized to the mitochondrial matrix and form complexes with mtHsp70. (A) U2OS cells were homogenized, and mitochondria were isolated. Mitochondria were swelled in hypotonic buffer to burst the outer mitochondrial membrane. Samples went untreated or were treated with proteinase K before or after sonication, which disrupts the mitochondrial inner membrane. Samples were analyzed by immunoblot for the presence of matrix-localized mtHsp70, hTid-1, and the mitochondrial inner membrane protein COx2. COx2 is digested before sonication, indicating that the inner mitochondrial membrane is exposed to protease. hTid-1L, hTid-1S, and mtHsp70 are only digested when proteinase K is added after the mitochondrial inner membrane is disrupted by sonication. (B) Digitonin extraction of mitochondrial proteins. Mitochondria were isolated from SAOS-2 cells and treated with the indicated amount of digitonin. The intermembrane space protein cytochrome c (cyto. c), and the integral inner membrane protein COx1 are extracted from the mitochondria, whereas hTid-1L and hTid-1S are held in the pellet by a digitonin-resistant membrane. (C) Endogenous hTid-1L and hTid-1S coimmunoprecipitate with mtHsp70. Immunoprecipitation experiments were performed from U20S cells by using mAbs specific for either hTid-1, mtHsp70, or Hsc70. Immune complexes were analyzed by SDS/PAGE and Western blot with hTid-1-, mtHsp70-, or Hsc70-specific mAbs.