Abstract
Background: The presence of "anti-DNA antibodies in abnormal titres" is a well established criterion for SLE classification, but there is no agreement on the performance of this test.
Objective: To study the correlation between clinical findings and five different solid and solution phase anti-DNA antibody assays.
Methods: 158 consecutively collected ANA positive sera were studied in a double blind fashion. Anti-DNA antibodies were determined by different solid phase assays (ssDNA-, dsDNA- specific ELISA, EliA anti-dsDNA assay, Crithidia luciliae assay), and by an experimental solution phase anti-DNA assay using biotinylated pUC18 plasmid, human, calf thymus, and E coli DNA. Antibody affinity was determined by surface plasmon resonance. Clinical data were obtained independently of the laboratory analyses and later related to the anti-dsDNA findings.
Results: Anti-dsDNA antibodies were most frequently detected by ELISA, but were not specific for SLE as they were present in up to 30% of other disease groups. Those detected by the Crithidia luciliae assay were predictive for SLE, while antibodies binding in solution phase ELISA using the pUC18 correlated strongly with the Crithidia luciliae assay. Surface plasmon resonance analysis showed that antibody binding to pUC18 was not due to higher relative affinity for dsDNA in general, but apparently to specificity for that plasmid DNA. Serum samples from three patients with lupus nephritis were positive in both pUC18 solution phase and Crithidia luciliae assays.
Conclusions: Assay principle selection is decisive for the detection of clinically significant anti-DNA antibodies. Revision of the anti-DNA antibody criterion in the SLE classification may be needed.
Full Text
The Full Text of this article is available as a PDF (558.0 KB).
Figure 1 .
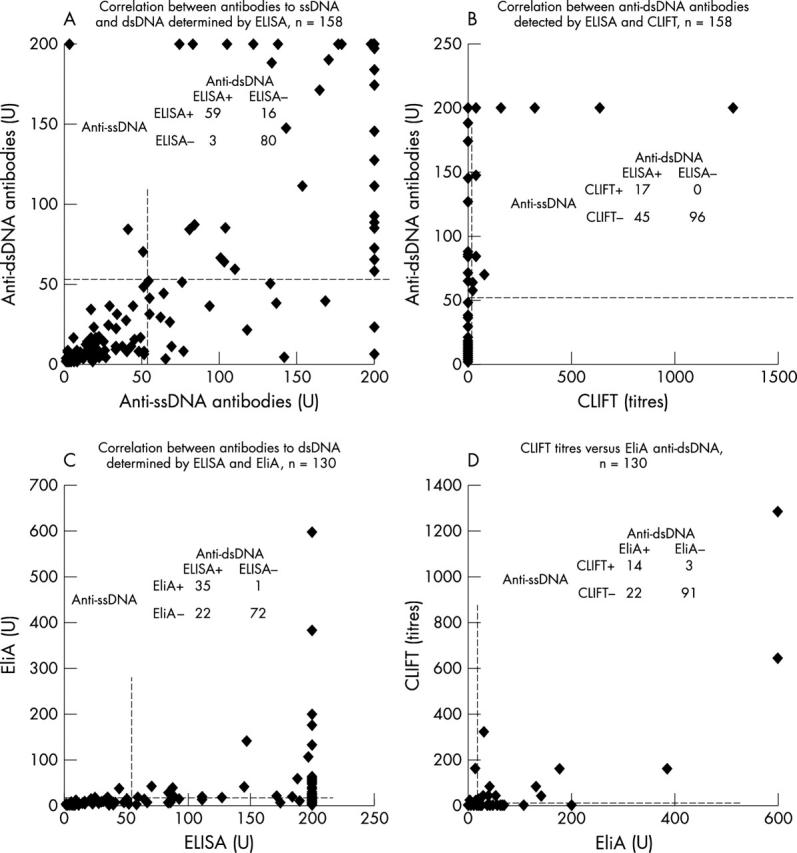
Consecutively collected ANA positive sera were examined for anti-DNA antibodies by different solid phase assays. Correlations are shown for anti-ssDNA and anti-dsDNA as detected by solid phase ELISA (rs = 0.4, p = 0.002) (A); anti-dsDNA antibodies detected in dsDNA ELISA versus CLIFT (rs = 0.11, p = 0.4) (B); dsDNA ELISA versus EliA dsDNA assay (rs = 0.55, p = 0.001) (C); and CLIFT versus EliA dsDNA assays (rs = 0.58, p<0.0001) (D). Inserted in each figure (A–D) are 2x2 tables showing the number of patients. The cut off values are 55 (U), 20 (U), and 10 (titre), for ELISA ssDNA/dsDNA, EliA dsDNA and CLIFT, respectively.
Figure 2 .
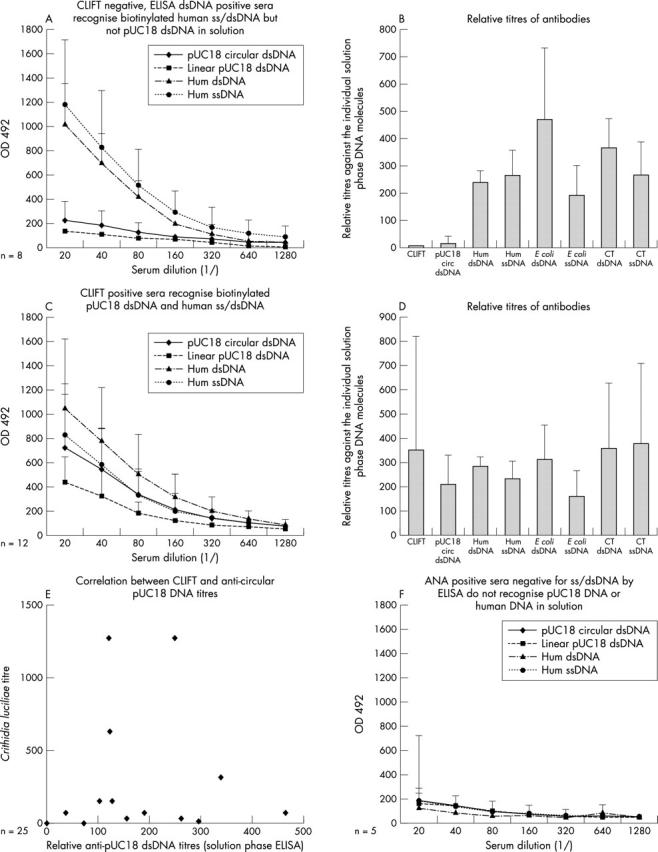
Groups of sera, selected as ELISA anti-dsDNA antibody positive, CLIFT negative (n = 8, A, B); anti-dsDNA antibodies positive in ELISA as well as in CLIFT (n = 12, C-E); or ANA positive, anti-ssDNA/dsDNA antibody negative sera (n = 5, F), were analysed in solution phase, biotinylated DNA ELISA (SPADE) using human, CT, E coli ssDNA/dsDNA or circular pUC18 dsDNA as antigens. Mean OD 490 (SD) for each group of sera at each serum dilution is presented for antibody binding to human dsDNA and ssDNA, and pUC18 circular and linear dsDNA (A, C, F). Correlation between CLIFT and relative pUC18 titres in all 25 sera included in these analyses is presented (E). Mean titres (SD) of anti-DNA antibodies from CLIFT negative (B) and CLIFT positive (D) sera against circular pUC18 dsDNA, human, calf thymus or E coli ssDNA/dsDNA demonstrate that in CLIFT positive sera, but not negative ones, antibodies are detected that recognise structures unique to pUC18 dsDNA (B, D).
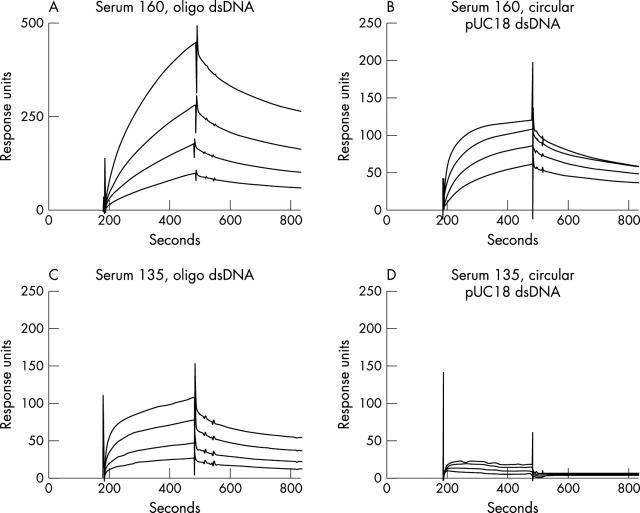
Figure 3 .
Surface plasmon resonance sensograms obtained at different concentrations of IgG anti-DNA antibodies interacting with immobilised 32 bp oligonucleotide or circular pUC18 dsDNA. IgG anti-DNA antibodies from CLIFT positive serum 160 binds both DNA ligands, while those from CLIFT negative serum 135 bind the oligonucleotide, but not pUC18. See table 1 for extended data, and "Materials and methods" for experimental details.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreassen Kristin, Bendiksen Signy, Kjeldsen Elisabeth, Van Ghelue Marijke, Moens Ugo, Arnesen Egil, Rekvig Ole Petter. T cell autoimmunity to histones and nucleosomes is a latent property of the normal immune system. Arthritis Rheum. 2002 May;46(5):1270–1281. doi: 10.1002/art.10254. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Boackle S. A., Holers V. M., Chen X., Szakonyi G., Karp D. R., Wakeland E. K., Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001 Nov;15(5):775–785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- Chan O. T., Madaio M. P., Shlomchik M. J. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999 Oct 1;163(7):3592–3596. [PubMed] [Google Scholar]
- D'Andrea D. M., Coupaye-Gerard B., Kleyman T. R., Foster M. H., Madaio M. P. Lupus autoantibodies interact directly with distinct glomerular and vascular cell surface antigens. Kidney Int. 1996 May;49(5):1214–1221. doi: 10.1038/ki.1996.175. [DOI] [PubMed] [Google Scholar]
- Desai D. D., Krishnan M. R., Swindle J. T., Marion T. N. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993 Aug 1;151(3):1614–1626. [PubMed] [Google Scholar]
- Eaton R. B., Schnneider G., Schur P. H. Enzyme immunoassay for antibodies to native DNA. Specificity and quality of antibodies. Arthritis Rheum. 1983 Jan;26(1):52–62. doi: 10.1002/art.1780260109. [DOI] [PubMed] [Google Scholar]
- Fields M. L., Sokol C. L., Eaton-Bassiri A., Seo S., Madaio M. P., Erikson J. Fas/Fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J Immunol. 2001 Aug 15;167(4):2370–2378. doi: 10.4049/jimmunol.167.4.2370. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Fredriksen K., Dahl B., Traavik T., Rekvig O. P. Inoculation with BK virus may break immunological tolerance to histone and DNA antigens. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8171–8175. doi: 10.1073/pnas.85.21.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J., Bleyman M., Rauch C. A., Kitchin P. A., Englund P. T. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell. 1986 Aug 29;46(5):717–724. doi: 10.1016/0092-8674(86)90347-8. [DOI] [PubMed] [Google Scholar]
- Hernando Monserrat, González Concepción, Sánchez Angel, Guevara Paloma, Navajo José Alejandro, Papisch Wolfgang, González-Buitrago José Manuel. Clinical evaluation of a new automated anti-dsDNA fluorescent immunoassay. Clin Chem Lab Med. 2002 Oct;40(10):1056–1060. doi: 10.1515/CCLM.2002.185. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ohyama T. Adjacent upstream superhelical writhe influences an Escherichia coli promoter as measured by in vivo strength and in vitro open complex formation. J Mol Biol. 1995 Dec 8;254(4):566–578. doi: 10.1006/jmbi.1995.0639. [DOI] [PubMed] [Google Scholar]
- Hoch S., Schwaber J. Specificity analysis of human anti-DNA antibodies. J Immunol. 1986 Feb 1;136(3):892–897. [PubMed] [Google Scholar]
- Isenberg D. A. Autoantibodies: markers of disease or pathogenic? Ann N Y Acad Sci. 1997 Aug 14;823:256–262. doi: 10.1111/j.1749-6632.1997.tb48398.x. [DOI] [PubMed] [Google Scholar]
- Isenberg D. A., Dudeney C., Williams W., Addison I., Charles S., Clarke J., Todd-Pokropek A. Measurement of anti-DNA antibodies: a reappraisal using five different methods. Ann Rheum Dis. 1987 Jun;46(6):448–456. doi: 10.1136/ard.46.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Kadlubowski M., Jackson M., Yap P. L., Neill G. Lack of specificity for antibodies to double stranded DNA found in four commercial kits. J Clin Pathol. 1991 Mar;44(3):246–250. doi: 10.1136/jcp.44.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karush F. Multivalent binding and functional affinity. Contemp Top Mol Immunol. 1976;5:217–228. doi: 10.1007/978-1-4684-8142-6_8. [DOI] [PubMed] [Google Scholar]
- Kavanaugh Arthur F., Solomon Daniel H., American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum. 2002 Oct 15;47(5):546–555. doi: 10.1002/art.10558. [DOI] [PubMed] [Google Scholar]
- Kojima Hidefumi, Gu Hua, Nomura Saeko, Caldwell Charles C., Kobata Tetsuji, Carmeliet Peter, Semenza Gregg L., Sitkovsky Michail V. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2170–2174. doi: 10.1073/pnas.052706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M. R., Jou N. T., Marion T. N. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol. 1996 Sep 15;157(6):2430–2439. [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaio M. P., Shlomchik M. J. Emerging concepts regarding B cells and autoantibodies in murine lupus nephritis. B cells have multiple roles; all autoantibodies are not equal. J Am Soc Nephrol. 1996 Mar;7(3):387–396. doi: 10.1681/ASN.V73387. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Fatenejad S., Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994 Feb 1;152(3):1453–1461. [PubMed] [Google Scholar]
- Marion T. N., Krishnan M. R., Desai D. D., Jou N. T., Tillman D. M. Monoclonal anti-DNA antibodies: structure, specificity, and biology. Methods. 1997 Jan;11(1):3–11. doi: 10.1006/meth.1996.0381. [DOI] [PubMed] [Google Scholar]
- Marion T. N., Tillman D. M., Krishnan M. K., Desai D. D., Jou N. T., Ruff M. B. Immunoglobulin variable-region structures in immunity and autoimmunity to DNA. Tohoku J Exp Med. 1994 May;173(1):43–63. doi: 10.1620/tjem.173.43. [DOI] [PubMed] [Google Scholar]
- Moens U., Seternes O. M., Hey A. W., Silsand Y., Traavik T., Johansen B., Rekvig O. P. In vivo expression of a single viral DNA-binding protein generates systemic lupus erythematosus-related autoimmunity to double-stranded DNA and histones. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12393–12397. doi: 10.1073/pnas.92.26.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C., Alas E., Morel L., Yang P., Wakeland E. K. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998 Mar 15;101(6):1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L., Blenman K. R., Croker B. P., Wakeland E. K. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci U S A. 2001 Jan 23;98(4):1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napirei M., Karsunky H., Zevnik B., Stephan H., Mannherz H. G., Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000 Jun;25(2):177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- Nossent J. C., Huysen V., Smeenk R. J., Swaak A. J. Low avidity antibodies to dsDNA as a diagnostic tool. Ann Rheum Dis. 1989 Sep;48(9):748–752. doi: 10.1136/ard.48.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaussen E., Rekvig O. P. Screening tests for antinuclear antibodies (ANA): selective use of central nuclear antigens as a rational basis for screening by ELISA. J Autoimmun. 1999 Aug;13(1):95–102. doi: 10.1006/jaut.1999.0295. [DOI] [PubMed] [Google Scholar]
- Pollard K. M., Webb J. Structural requirements of DNA used in the Farr assay to detect antibodies directed against double-stranded DNA. Rheumatol Int. 1987;7(4):161–168. doi: 10.1007/BF00270364. [DOI] [PubMed] [Google Scholar]
- Radic M. Z., Seal S. N. Selection of recurrent V genes and somatic mutations in autoantibodies to DNA. Methods. 1997 Jan;11(1):20–26. doi: 10.1006/meth.1996.0383. [DOI] [PubMed] [Google Scholar]
- Raz E., Brezis M., Rosenmann E., Eilat D. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989 May 1;142(9):3076–3082. [PubMed] [Google Scholar]
- Rekvig O. P., Fredriksen K., Hokland K., Moens U., Traavik T., Krishnan M. R., Marion T. Molecular analyses of anti-DNA antibodies induced by polyomavirus BK in BALB/c mice. Scand J Immunol. 1995 Jun;41(6):593–602. doi: 10.1111/j.1365-3083.1995.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Moens U., Sundsfjord A., Bredholt G., Osei A., Haaheim H., Traavik T., Arnesen E., Haga H. J. Experimental expression in mice and spontaneous expression in human SLE of polyomavirus T-antigen. A molecular basis for induction of antibodies to DNA and eukaryotic transcription factors. J Clin Invest. 1997 Apr 15;99(8):2045–2054. doi: 10.1172/JCI119373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekvig O. P., Nossent J. C. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis Rheum. 2003 Feb;48(2):300–312. doi: 10.1002/art.10739. [DOI] [PubMed] [Google Scholar]
- Smeenk R. J., van den Brink H. G., Brinkman K., Termaat R. M., Berden J. H., Swaak A. J. Anti-dsDNA: choice of assay in relation to clinical value. Rheumatol Int. 1991;11(3):101–107. doi: 10.1007/BF00304496. [DOI] [PubMed] [Google Scholar]
- Smeenk R., van der Lelij G., Swaak T., Groenwold J., Aarden L. Specificity in systemic lupus erythematosus of antibodies to double-stranded DNA measured with the polyethylene glycol precipitation assay. Arthritis Rheum. 1982 Jun;25(6):631–638. doi: 10.1002/art.1780250605. [DOI] [PubMed] [Google Scholar]
- Somerfield S. D., Roberts M. W., Booth R. J. Double-stranded DNA antibodies: a comparison of four methods of detection. J Clin Pathol. 1981 Sep;34(9):1032–1035. doi: 10.1136/jcp.34.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Immunochemistry of DNA. Int Rev Immunol. 1989;5(1):1–22. doi: 10.3109/08830188909086987. [DOI] [PubMed] [Google Scholar]
- Swaak A. J., Groenwold J., Aarden L. A., Feltkamp T. E. Detection of anti-dsDNA as diagnostic tool. Ann Rheum Dis. 1981 Feb;40(1):45–49. doi: 10.1136/ard.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tzioufas A. G., Terzoglou C., Stavropoulos E. D., Athanasiadou S., Moutsopoulos H. M. Determination of anti-ds-DNA antibodies by three different methods: comparison of sensitivity, specificity and correlation with lupus activity index (LAI). Clin Rheumatol. 1990 Jun;9(2):186–192. doi: 10.1007/BF02031967. [DOI] [PubMed] [Google Scholar]
- Van Bruggen M. C., Kramers C., Berden J. H. Autoimmunity against nucleosomes and lupus nephritis. Ann Med Interne (Paris) 1996;147(7):485–489. [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Ward M. M., Pisetsky D. S., Christenson V. D. Antidouble stranded DNA antibody assays in systemic lupus erythematosus: correlations of longitudinal antibody measurements. J Rheumatol. 1989 May;16(5):609–613. [PubMed] [Google Scholar]
- Westhoff C. M., Whittier A., Kathol S., McHugh J., Zajicek C., Shultz L. D., Wylie D. E. DNA-binding antibodies from viable motheaten mutant mice: implications for B cell tolerance. J Immunol. 1997 Sep 15;159(6):3024–3033. [PubMed] [Google Scholar]
- Williams W. M., Isenberg D. A. A cross-sectional study of anti-DNA antibodies in the serum and IgG and IgM fraction of healthy individuals, patients with systemic lupus erythematosus and their relatives. Lupus. 1996 Dec;5(6):576–586. doi: 10.1177/096120339600500604. [DOI] [PubMed] [Google Scholar]
- Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin North Am. 1989 Feb;15(1):1–18. [PubMed] [Google Scholar]
- Yoh K., Itoh K., Enomoto A., Hirayama A., Yamaguchi N., Kobayashi M., Morito N., Koyama A., Yamamoto M., Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001 Oct;60(4):1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]