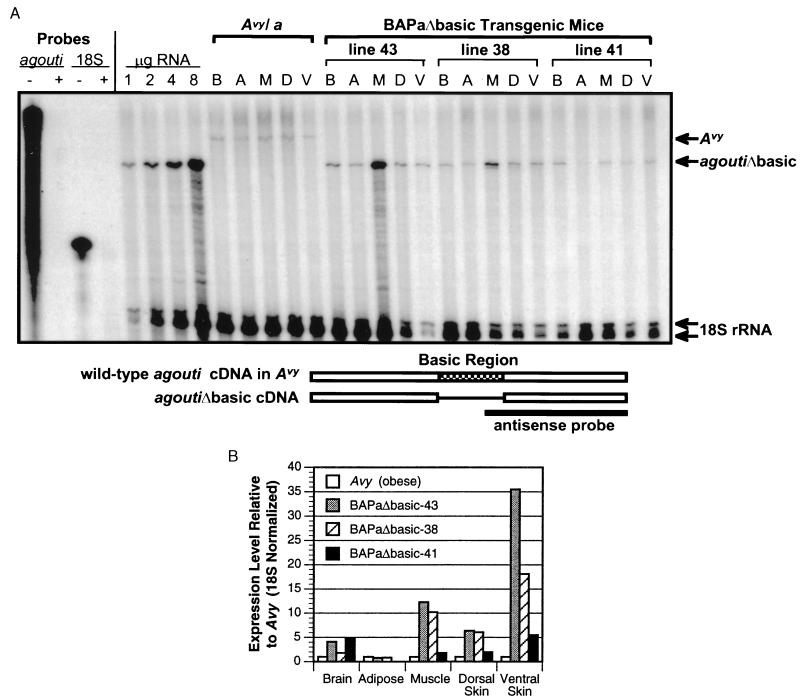
Figure 2.
Analysis of transgene expression by RPA. (A) Quantitative RNase protection was performed with 4 μg of total RNA from brain (B), epididymal adipose (A), skeletal muscle (M), and dorsal (D) and ventral (V) skin of 30- to 40-week-old adult male mice that carry either the Avy/a mutation (solid yellow fur and obese; C57BL/6J strain) or one copy of the BAPaΔbasic transgene (Tg/−; FVB/N stock). Antisense probes specific for agouti exon 4 (165 ng) or 18S rRNA (320 ng) were hybridized separately with yeast tRNA and digested with (+) or without (−) RNase to show complete digestion of the probe in the absence of specific target sequences (lanes 1–4 at left). In all other samples, both probes were hybridized simultaneously with total tissue RNA followed by RNase digestion. Increasing amounts (1–8 μg) of total muscle RNA from BAPaΔbasic-43 Tg mice yielded quantitative protection of both agouti and 18S transcripts, showing a molar excess of each probe relative to the range of specific target sequences used throughout the experiment. The relative positions of protected bands corresponding to ectopic agouti in Avy/a, agoutiΔbasic in the Tg lines and 18S in all samples are indicated by arrows at the right. The region of agouti to which the agouti antisense probe hybridizes is indicated by the schematic at the bottom. (B) The level of agouti-specific RNA detected in the RPA shown in A was normalized to the level of 18S rRNA detected in the same tissue. The 18S-normalized values for agoutiΔbasic expression in BAPaΔbasic Tg mice were then divided by the amount of 18S-normalized ectopic agouti expression in the same tissues of Avy/a mice.